The electrochemical reduction of carbon dioxide (CO2) presents an innovative opportunity for addressing climate change while simultaneously creating valuable chemicals. Efforts in this domain have primarily focused on improving catalyst design, yet the influence of electrolyte composition on product selectivity remains an underexplored territory. A recent study sheds light on this critical aspect, revealing that varying the electrolyte can pivot the outcomes of CO2 reduction reactions.
Innovation Through Metal-Organic Frameworks
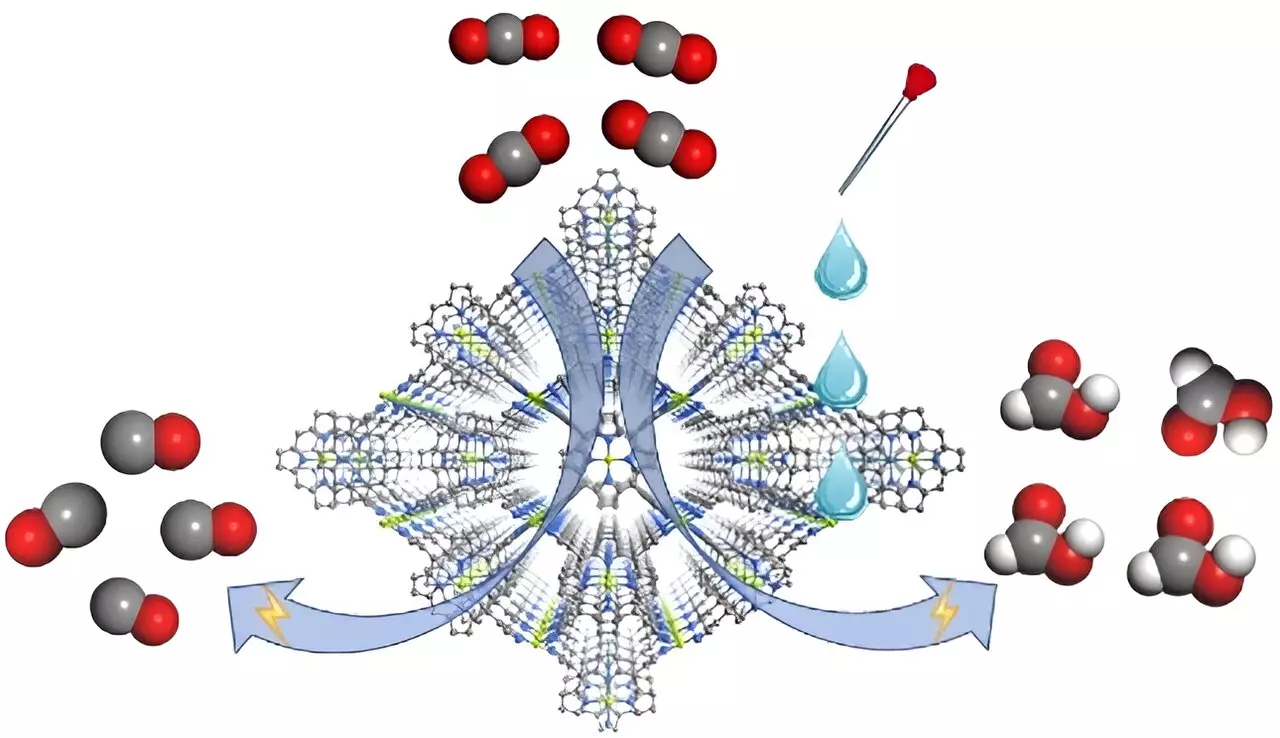
Research conducted by a team led by Prof. Cao Rong and Prof. Zhang Teng, published in Angewandte Chemie International Edition, introduces a groundbreaking metal-organic framework (MOF) electrocatalyst called FICN-8. Created using Cu(porphyrin)-derived ligands combined with Cu(pyrazolate) units, this three-dimensional catalyst structure allows for enhanced accessibility of catalytic sites, thereby bolstering its electrochemical performance. By employing FICN-8, the researchers demonstrated significant control over the selectivity of CO2 reduction products by simply adjusting the composition of the electrolyte.
The study highlights that in a specific electrolyte composed of tetrabutylammonium hexafluorophosphate (TBAPF6) and acetonitrile (MeCN), FICN-8 exhibited remarkable activity, achieving a CO production selectivity of up to 95%. However, when water or trifluoroethanol (TFE) was introduced as a proton source, a remarkable transformation occurred. The primary product shifted from carbon monoxide (CO) to formic acid, with an optimum Faradaic efficiency of 48% for formic acid achieved by incorporating 2.65 mol/L of water or 0.55 mol/L of TFE into the electrolyte. This finding underscores the pivotal role that electrolyte composition plays in directing the reaction pathways of CO2 reduction.
Understanding Reaction Mechanisms
To delve deeper into the mechanics underlying the observed selectivity transition, the researchers conducted kinetic isotope effect (KIE) measurements, which revealed varying kinetic behaviors for CO and formic acid production. A KIE value close to unity for CO production contrasted sharply with a larger KIE of 3.7 ± 0.7 for formic acid. This divergence indicates a direct integration of protons in the formation of formic acid, suggesting that shifting products is intricately linked to the presence of protons in the reaction milieu.
Theoretical investigations highlighted that the crucial step in formic acid synthesis is the reductive adsorption of hydride at the porphyrin nitrogen site, while CO is produced through a pathway that is largely independent of proton concentration. This revelation illuminates the necessity of investigating not just catalyst properties but also the intricate interplay with electrolyte environments to optimize CO2 reduction processes.
The study effectively emphasizes the often-overlooked importance of electrolyte composition in the selectivity of electrochemical CO2 reduction. It not only paves the way for the development of novel catalyst-electrolyte systems but also opens up new avenues for research in sustainable chemical production, reinforcing the potential of CO2 as a valuable resource rather than a waste product.

Leave a Reply