Recent advancements in chemistry have shed light on the behavior of metal complexes as potential catalysts for numerous chemical reactions. A breakthrough study led by Professor Jaeheung Cho from UNIST’s Department of Chemistry has unveiled significant insights into the interactions between cobalt(III)-based metal complexes and nitrile compounds. Published in the esteemed Journal of the American Chemical Society, this research holds promising implications for drug development, particularly in the realm of anticancer therapies.
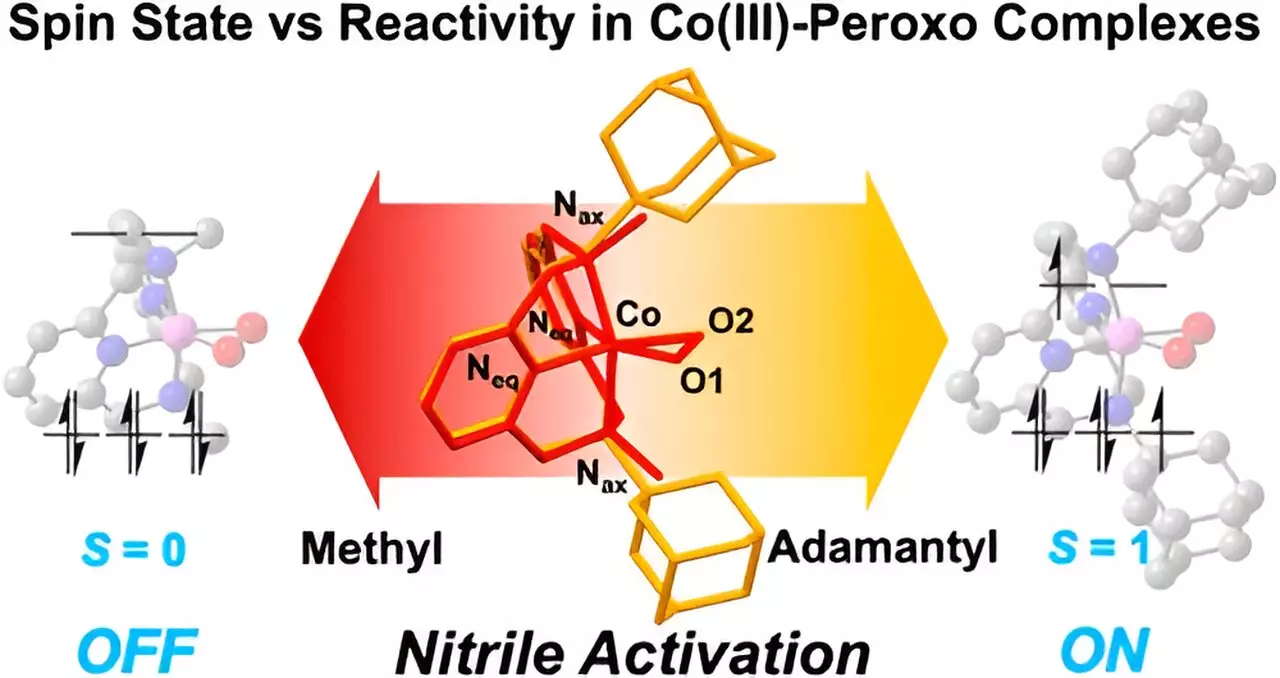
Nitriles are a prevalent class of organic compounds utilized extensively in pharmaceuticals and agricultural pesticides. However, their inherent reactivity poses challenges that can hinder effective application. The research team focused on the activation mechanism of nitriles within biomimetic frameworks by employing cobalt(III) complexes, emphasizing the critical role of metal spin states in dictating reaction efficacy.
The study revealed that even minor alterations in metal properties could significantly influence the speed and outcomes of reactivity. This observation is pivotal as it directs attention to the underlying principles governing reaction mechanisms in metal complexes, potentially guiding scientists in designing more effective catalysts for various applications.
To facilitate their investigation, the researchers utilized a novel structure known as the “Macrocyclic Pyridinophane System,” a versatile platform that allows for fine-tuning the molecular architecture of cobalt compounds. This framework enabled the team to systematically explore the relationship between the size of functional groups attached to cobalt complexes and their resulting reactivity with nitriles.
Remarkably, compounds featuring larger adamantyl groups exhibited enhanced activation of nitriles compared to their smaller methyl counterparts, which displayed negligible reactivity. This phenomenon can be attributed to the variations in spin states influenced by the size of the attached groups, underscoring the importance of molecular design in chemical interactions.
One of the standout findings of this research is the successful reaction of cobalt(III)-peroxo species with nitriles at ambient temperature, leading to the formation of a compound with promising anticancer properties. This advancement could bridge the gap between basic research and practical pharmaceutical applications, providing a foundation for developing novel drugs targeting cancer.
First author Seonghan Kim articulated this potential succinctly, stating, “We successfully synthesized cobalt(III)-peroxo species with varying spin states by modifying the three-dimensional configuration of the ligands, revealing a close relationship with nitrile reactivity.” This statement emphasizes the significance of ligand architecture in influencing the behavior of cobalt complexes and, by extension, the possibilities for new therapeutic agents.
This research not only enhances our understanding of cobalt(III)-based metal complexes but also sets the stage for future explorations in drug development. The demonstrated link between ligand design and nitrile reactivity reinforces the need for continued innovation in coordinating chemistry. As researchers delve deeper into the complexities of metal interactions, we may witness the emergence of new, effective strategies in the fight against diseases, heightening the impact of these findings within the broader scientific community.

Leave a Reply