Recent advancements in the study of solvation shells have opened new avenues for understanding ionic behavior in solutions. Researchers from notable institutions, including the Fritz Haber Institute, Sorbonne University, and Uppsala University, have published a groundbreaking study in *Nature Communications* that details their innovative approach to probing these elusive structures. Solvation shells—the enveloping layers of solvent molecules around dissolved particles—play a crucial role in determining the chemical and physical properties of solutions. However, the intricate nature of these shells has long posed a challenge for scientists seeking to analyze their formation and behavior.
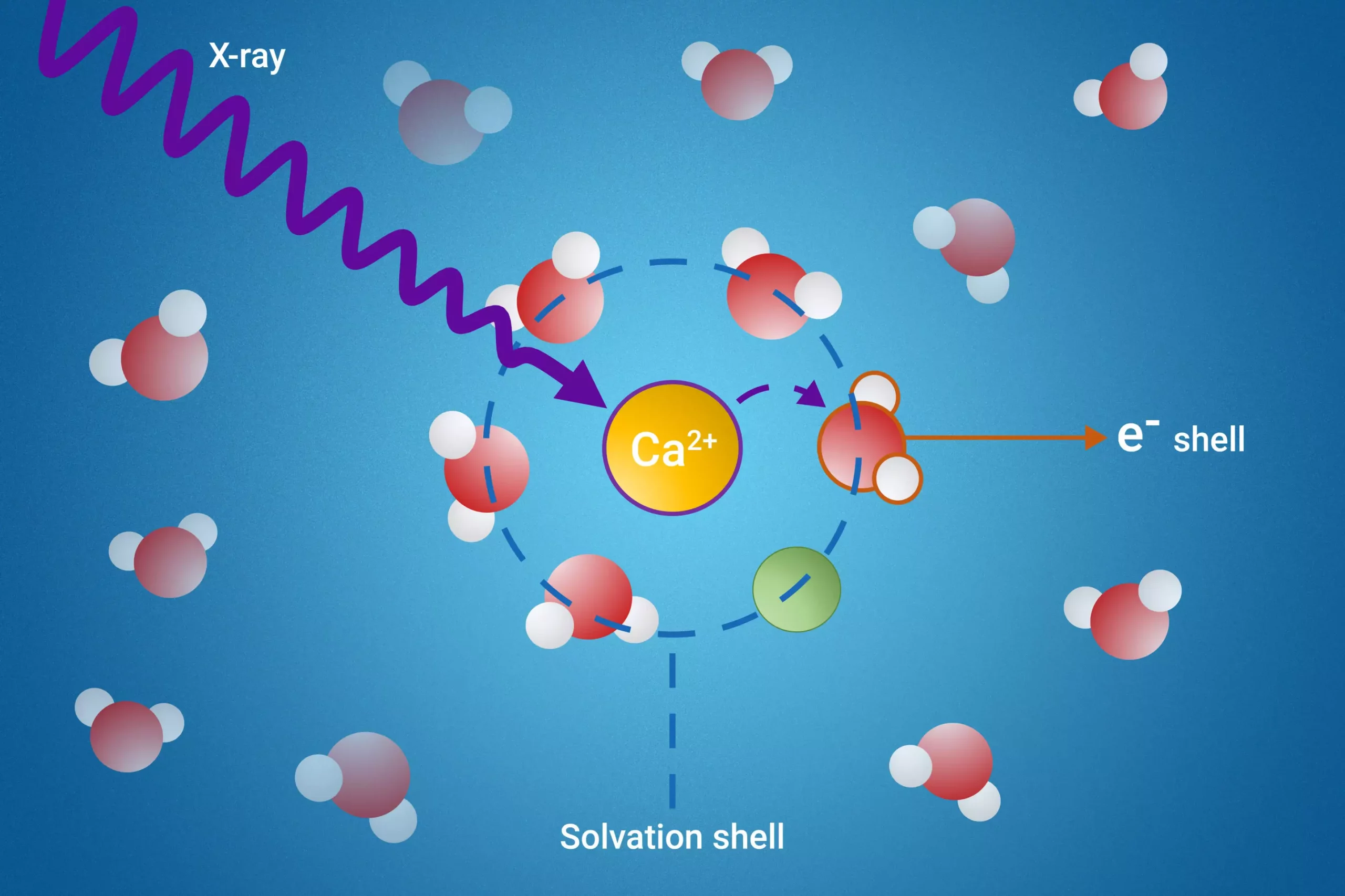
At the heart of this study lies a novel technique known as resonant intermolecular Coulombic decay (ICD). This method allows researchers to observe interactions among solvent molecules at a near-molecular level. By using X-ray excitation to probe the solvation shells, scientists can closely monitor how solvent molecules behave in the transient period during which they interact with their neighbors. This ability to target and analyze specific molecules within a complex environment represents a significant leap forward in the study of solvation dynamics.
One of the pivotal discoveries made by the research team was that a specific process of ICD serves as an excellent indicator of ion pair formation. This finding is particularly noteworthy given the complexities associated with isolating the behaviors of molecules in solvation shells. The ability to measure electron binding energies of water molecules residing within the first solvation shell further underscores the study’s significance, as these measurements were previously out of reach for researchers.
The implications of this research extend far beyond the confines of basic science; understanding solvation shells is integral to a myriad of disciplines, including chemistry, biology, and materials science. For chemists, insights into ion behavior can enhance the development of more effective catalysts and materials. Biologists stand to benefit from a deeper comprehension of cellular processes involving ion transport, while atmospheric scientists may refine their models for predicting weather patterns and chemical reactions in the environment.
The versatility of this new probing technique means it is likely to have a far-reaching impact across diverse scientific domains. For example, in electrochemistry, improved understanding of solvation dynamics can lead to advancements in battery technology and energy-storage solutions. Additionally, in the realm of materials science, manipulating solvation shell properties could result in the creation of novel materials with tailored characteristics.
The exploration of solvation shells through the lens of resonant intermolecular Coulombic decay represents a pivotal advancement in our understanding of ionic behavior in solutions. As researchers continue to refine and apply these methods, the future of scientific inquiry into solvation dynamics appears promising, potentially leading to breakthroughs that resonate through multiple fields.

Leave a Reply