Hydrogen (H2) has long been considered a promising fuel for reducing greenhouse gases, especially when produced by splitting water molecules (H2O) using renewable energy sources. However, the process of breaking water into hydrogen and oxygen is not as simple as it may seem. Two simultaneous electrochemical reactions take place, each requiring specific catalysts to facilitate the breaking and remaking of chemical bonds.
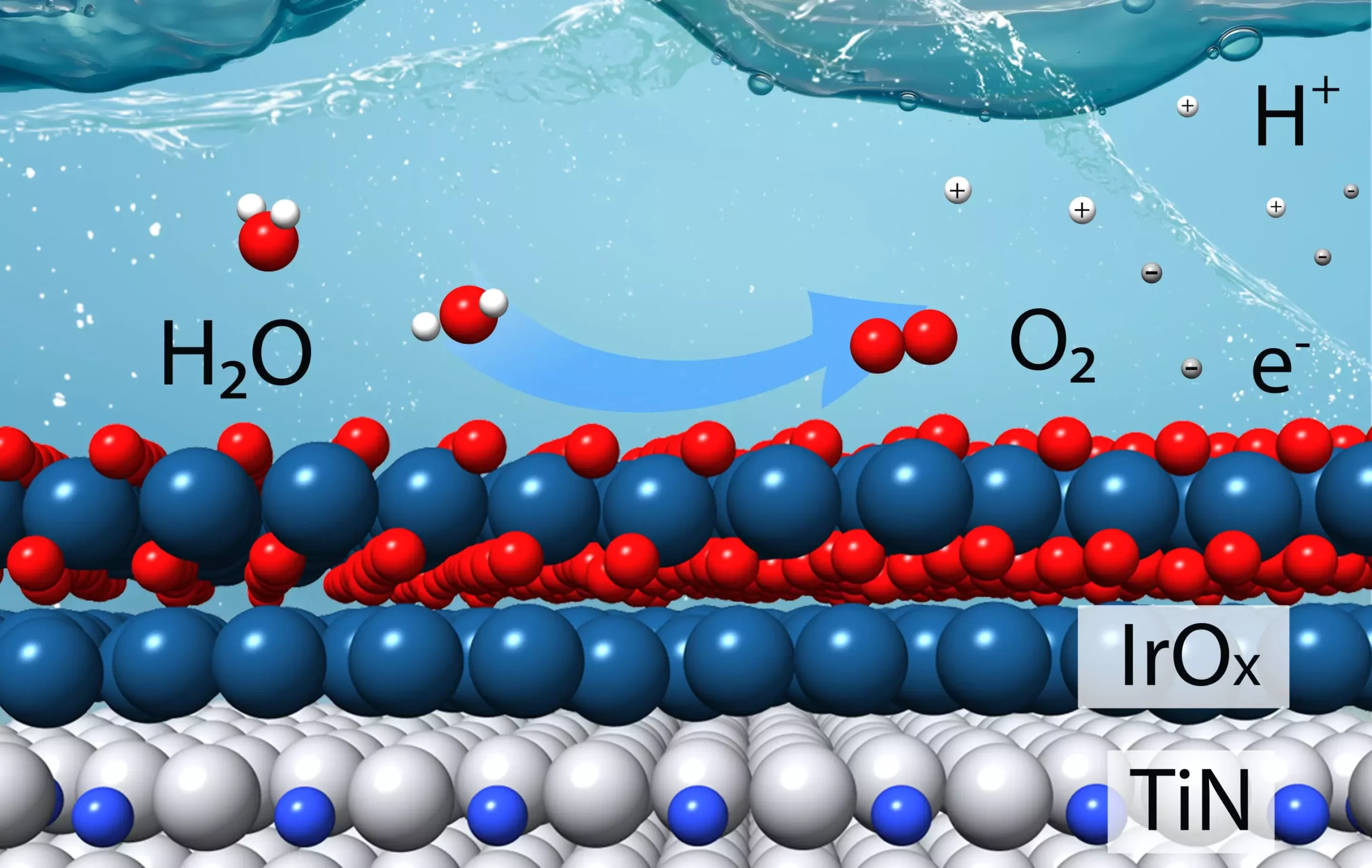
Recently, scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and Columbia University have made significant progress in developing a new efficient catalyst for the oxygen evolution reaction – the more challenging part of the water-splitting process. This catalyst was meticulously designed based on theoretical calculations aimed at minimizing the use of iridium, an expensive metal commonly used as a catalytic material, while maximizing stability in acidic conditions. The experimental results confirmed the efficacy of the new catalyst, showing it to be four times more efficient than the current state-of-the-art iridium catalyst in producing hydrogen.
Understanding Catalyst Design
The catalyst developed by the research team involves combining titanium with nitrogen to create stable “titanium nitrides” that can withstand acidic reaction conditions. The core of the catalyst consists of titanium nitride, with only a thin layer of iridium on the surface. The theoretical calculations conducted by the team helped determine the optimal number of iridium layers needed to enhance both performance and catalytic stability during the oxygen evolution reaction.
Experimental Validation
To validate the theoretical predictions, the team created thin films and powdered samples closely resembling the surfaces used in modeling calculations. Various techniques, including transmission electron microscopy and X-ray spectroscopy studies, were employed to study the thin films and nanoparticles. The characterization studies confirmed the strong interaction between iridium and titanium, which contributed to the stability and activity of the catalyst.
While the new catalyst has shown promising results in laboratory testing, scaling up production remains a challenge. The researchers emphasize the need for further improvements to optimize the consistency of the catalyst powders for industrial applications. By developing core-shell structures with a uniform layer of iridium, the cost of water splitting could be significantly reduced, bringing us closer to producing large quantities of green hydrogen at a lower cost.
The development of efficient catalysts for hydrogen production is crucial in transitioning to a more sustainable energy future. By combining theoretical insights with experimental validation, scientists can continue to refine catalyst design and optimize production processes to make green hydrogen production a viable and cost-effective solution for reducing greenhouse gas emissions.

Leave a Reply