The field of organic chemistry is witnessing significant developments aimed at enhancing sustainability and efficiency in chemical synthesis. Recently, chemists from the National University of Singapore (NUS) have achieved a groundbreaking innovation in the synthesis of trisubstituted Z-alkenes. This family of compounds is crucial due to their presence in numerous biologically active molecules and their importance in stereospecific reactions. Previous attempts to selectively produce Z-isomers have faced hurdles, primarily due to their inherent thermodynamic instability compared to their E counterparts. The NUS team, led by Associate Professor Koh Ming Joo, has developed a method which not only circumvents this challenge but also leverages the use of an affordable iron catalyst.
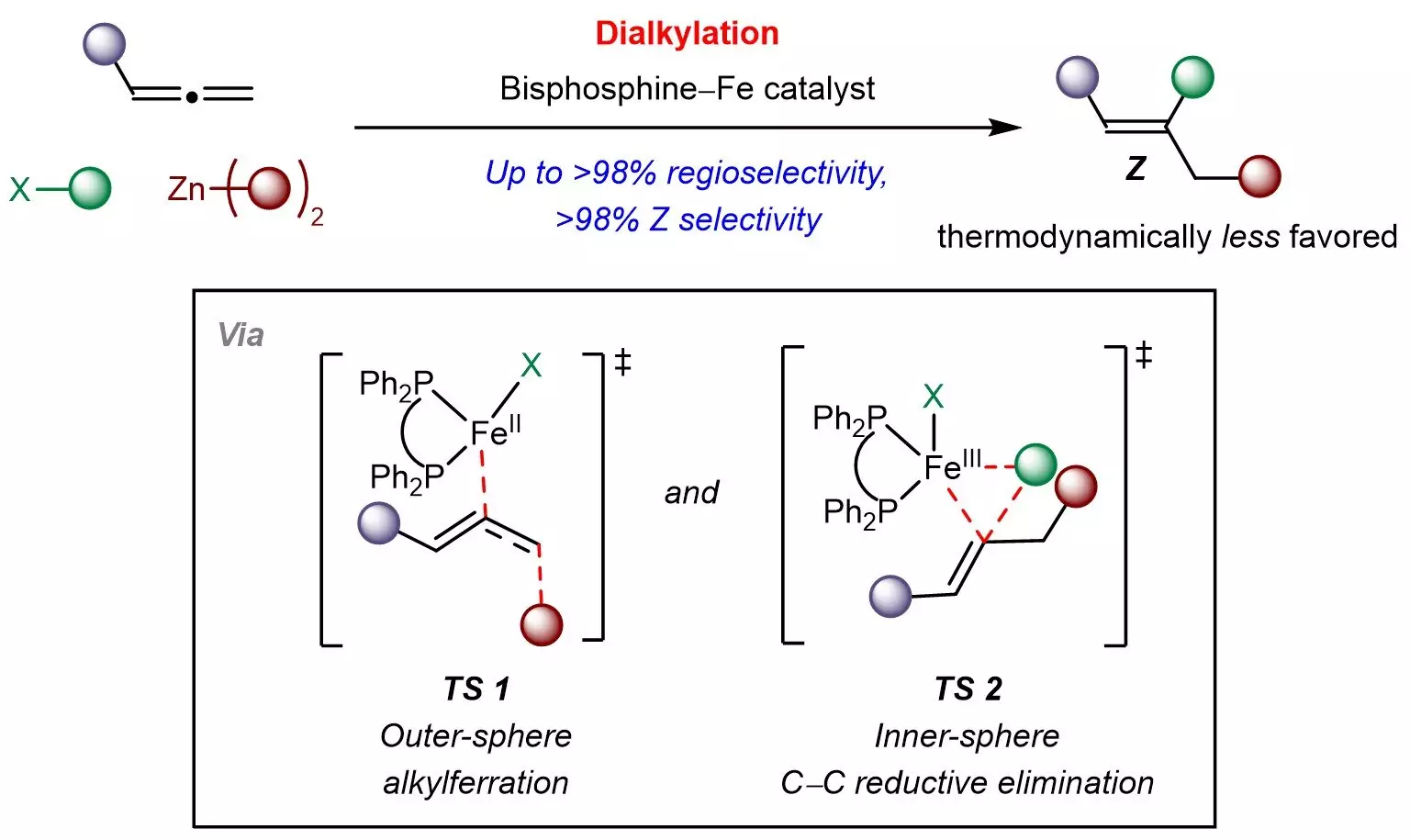
The newly developed method employs a bisphosphine-iron catalyst, which is notable for its abundance, low toxicity, and cost-effectiveness. This innovative approach facilitates the reaction between allenes and sp3-hybridized organohalides or organozinc reagents, enabling the selective introduction of alkyl groups into the allene structure. This multicomponent strategy is significant as it ensures controlled site and Z-selectivity, paving the way for the efficient synthesis of complex chemical compounds. This advancement not only simplifies the synthetic pathways but also aligns with the increasing demand for green chemistry practices where sustainability is prioritized.
One of the striking applications of this new method is its use in the synthesis of a glucosylceramide synthase inhibitor, a compound that requires the specific Z-configuration for effective bioactivity. This underscores the dual impact of the research—not only does it fill a critical gap in chemical literature, but it also enhances the potential for practical applications in drug development and discovery. As these trisubstituted Z-alkenes often serve as intermediates in the synthesis of pharmaceuticals, the ability to efficiently produce them can expedite the development of new medicinal compounds effectively.
The research further reveals a noteworthy mechanism termed outer-sphere radical-mediated alkylferration, which is followed by inner-sphere carbon-carbon bond formation. This insight is vital for scientists seeking to refine kinetically controlled reactions involving allenes and other π-systems. Such understanding not only propels the existing knowledge of reaction mechanisms but also sets the stage for designing advanced multicomponent transformations utilizing readily available raw materials.
Looking forward, the research team aims to expand on their findings by exploring additional multicomponent transformations. The goal is to convert common raw materials into high-value chemical products, thereby enhancing the economic viability of processes in various industries. As global emphasis on sustainability intensifies, methodologies that integrate environmental considerations with economic practicality will become increasingly essential.
The innovative approach put forth by the NUS team serves as a promising stride towards sustainable chemistry, with far-reaching implications for both academic research and practical industrial applications.

Leave a Reply