Recent developments in the field of molecular and coordination chemistry have opened new pathways for exploring oxidation potential. Leading this groundbreaking research, Professor Ingo Krossing from the University of Freiburg has pioneered methods that notably elevate the oxidation capabilities of positive ions like Ag+ and NO+, which are integral to various chemical processes. This research introduces an innovative approach utilizing weakly interacting solvents and anions to achieve unprecedented redox potentials. The implications of these findings, published in *Nature Communications*, could influentially reshape the methodologies in electrochemistry and materials science.
The study reported that traditional solvents impose significant limitations on the oxidation potential of positive ions. Generally, in standard conditions, positive ions exhibit oxidation potentials ranging from +0.65 to +1.0 V when assessed against the ferrocene reference (Fc+/0). However, Krossing and his team remarkably extended this range to potentials as high as +1.50 to +1.52 V, demonstrating not only an increase but an uncharted territory for chemical reactivity. This enhancement is pivotal as it means that harder-to-oxidize substrates may now become viable targets for redox reactions, considerably advancing the landscape of synthetic chemistry.
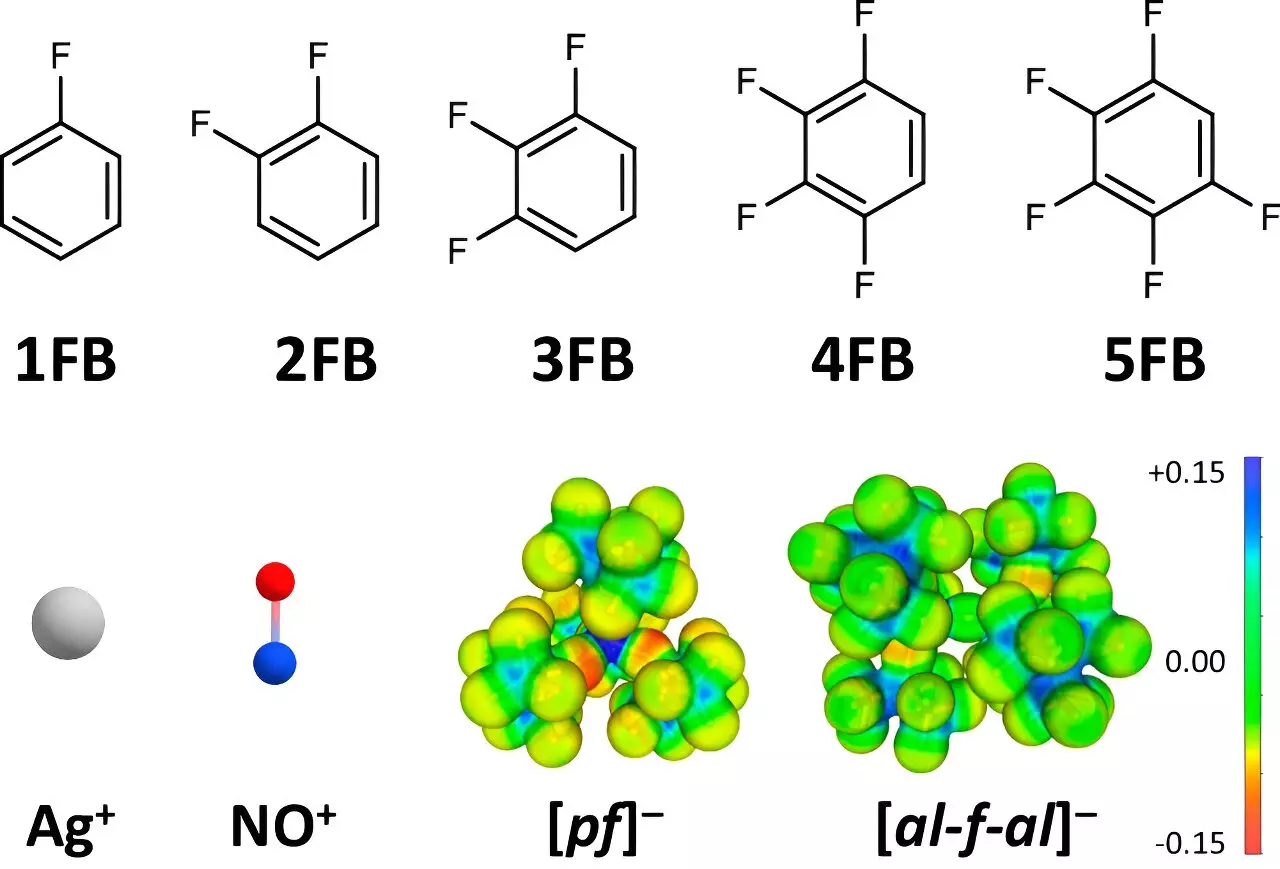
At the core of this advancement is the strategic selection of particularly weakly coordinating anions (WCA) and fluorinated benzene derivatives as solvent mediums. By carefully crafting the molecular environment, the researchers significantly mitigate the undesirable interactions between positive ions and their surroundings, which typically reduce their oxidative power. Dr. Johannes Hunger from the Max Planck Institute contributed crucial insights into the dielectric properties of these solvents, establishing their superiority over traditional options like dichloromethane and acetone. The findings revealed that solvents with two to four fluorine atoms exhibited substantially higher dielectric constants, allowing for an efficient stabilization of the positive ions while simultaneously lessening their interaction with the solvent.
Through meticulous techniques like single-crystal X-ray diffraction, the research team was able to elucidate the structural dynamics between the solvents and positive ions. This advanced analytical approach provided tangible evidence that the degree of fluorination can influence the strength of interactions. Dr. Malte Sellin, a co-author of the study, emphasized that as the number of fluorine substituents increased, the interaction with Ag+ and NO+ ions diminished, permitting their oxidation potential to rise significantly. The ability to retain these “almost undisturbed particles” is a crucial factor that enhances their effectiveness as oxidizing agents.
The implications of this research extend far beyond theoretical constructs, hinting at a multitude of applications in electrocatalysis and as redox mediators. The newfound potential for efficient electron transfer is poised to revolutionize the development of sustainable energy systems, particularly in the realm of rechargeable batteries and fuel cells, where oxidation-reduction reactions are integral to performance. Additionally, the enhanced oxidation capabilities could lead to novel chemical transformations that were previously deemed impossible, accelerating innovations in synthetic materials and organic chemistry.
Professor Krossing’s team’s discovery represents a significant leap in redox chemistry. By redefining the boundaries of oxidation potential through targeted solvent and anion design, this research not only establishes a new frontier in scientific inquiry but also sets the stage for practical applications that promise to tangibly impact various fields. As researchers continue to explore these complex interactions, the knowledge gleaned could foster an exciting era of chemical advancements, highlighting the importance of creativity and thorough investigative work in scientific progress. The unfolding narrative of oxidizing agents like Ag+ and NO+ has only begun, and its trajectory will undoubtedly chart new routes for both academic and industrial exploration.

Leave a Reply