The relentless search for sustainable energy solutions has led scientists to explore various technologies to mitigate carbon emissions, particularly focusing on the electrochemical reduction of carbon dioxide (CO2). This method harnesses electrical energy to transform captured CO2 into valuable products such as methanol and ethanol, thereby presenting a dual benefit of emission reduction and renewable fuel production. However, a significant barrier has been the development of efficient and adaptable catalysts that could make this process economically viable. Recent discoveries made by researchers from Brookhaven National Laboratory, in collaboration with Yale University and the University of North Carolina at Chapel Hill, signal a promising breakthrough in this area.
Catalysts play a critical role in enhancing the rate of chemical reactions with minimal energy input. In the context of CO2 reduction, effective catalysts not only need to promote fast reactions but must also work under conditions that allow their use on a larger, commercial scale. Traditional catalysts require considerable energy to function, making them unsuitable for widespread application. The work undertaken by the Brookhaven-led team represents a paradigm shift, identifying a catalyst that operates with significantly reduced energy requirements while dramatically increasing reaction speed.
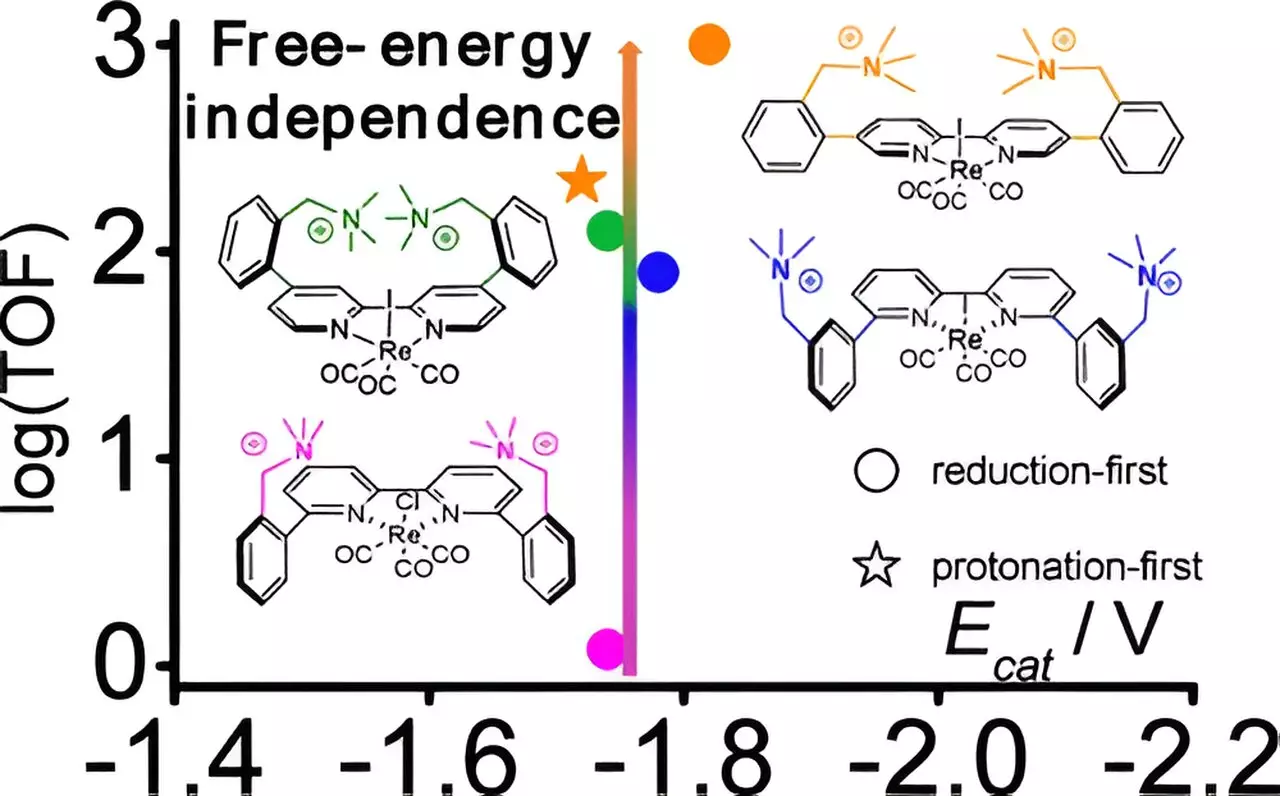
The researchers specifically focused on a type of catalyst based on the metal rhenium, known for its ability to facilitate the reduction of CO2. By innovating on existing designs, they created multiple versions of this catalyst, each modified by strategically placing positively charged molecules, or cations, at varying distances from the rhenium center. This minor adjustment proved profound—the catalyst’s performance improved by an astonishing factor of 800 due to optimized spatial arrangements that enhance reaction efficiency.
Delving into the mechanisms behind the improved catalysis, the research team employed advanced computational chemistry techniques. This analytical approach allowed them to understand how the placement of cations stabilizes the catalytic process, leading to the discovery of a previously unobserved low-energy reaction pathway for rhenium-based catalysts. Such insights are vital, as they can catalyze the design of more efficient catalysts not just for CO2 reduction but for various industrial applications.
Despite reliance on conventional catalytic frameworks, the impact of geometric adjustments highlighted by this research challenges existing paradigms, underscoring that minute changes can lead to substantial improvements in catalytic functionality. Through techniques such as cyclic voltammetry and infrared spectroelectrochemistry, which measure reaction dynamics and structural transformations, the team gathered robust data to support their findings.
Looking forward, the researchers are exploring ways to enhance their catalytic system. A promising avenue involves incorporating semiconductor light absorbers, such as silicon, into their design. By capturing sunlight and converting it into electrical energy, these materials could provide a supplementary energy source for the catalytic process, thus reducing reliance on external electrical inputs. This synergy between solar energy and electrochemical processes aligns perfectly with the overarching goals of sustainability and clean energy production.
The advancements made by the Brookhaven team not only represent a significant step in fostering efficient CO2 reduction mechanisms but also serve as a catalyst for future innovations. By integrating sophisticated materials and processes, these emerging technologies hold the potential to alter the landscape of renewable fuels.
The exploration of electrochemical reduction of CO2 is not merely a scientific endeavor; it embodies a critical response to global climate change challenges. By developing catalysts that minimize energy use and maximize efficiency, researchers are paving the way towards practical and sustainable energy solutions. The collaborative efforts within the Brookhaven research community exemplify how interdisciplinary approaches can accelerate breakthroughs in technology. As the world seeks to embrace greener alternatives, innovations in catalysis will undoubtedly play an integral role, possibly transforming CO2, a notorious pollutant, into valuable energy resources that could support a cleaner, more sustainable future.

Leave a Reply