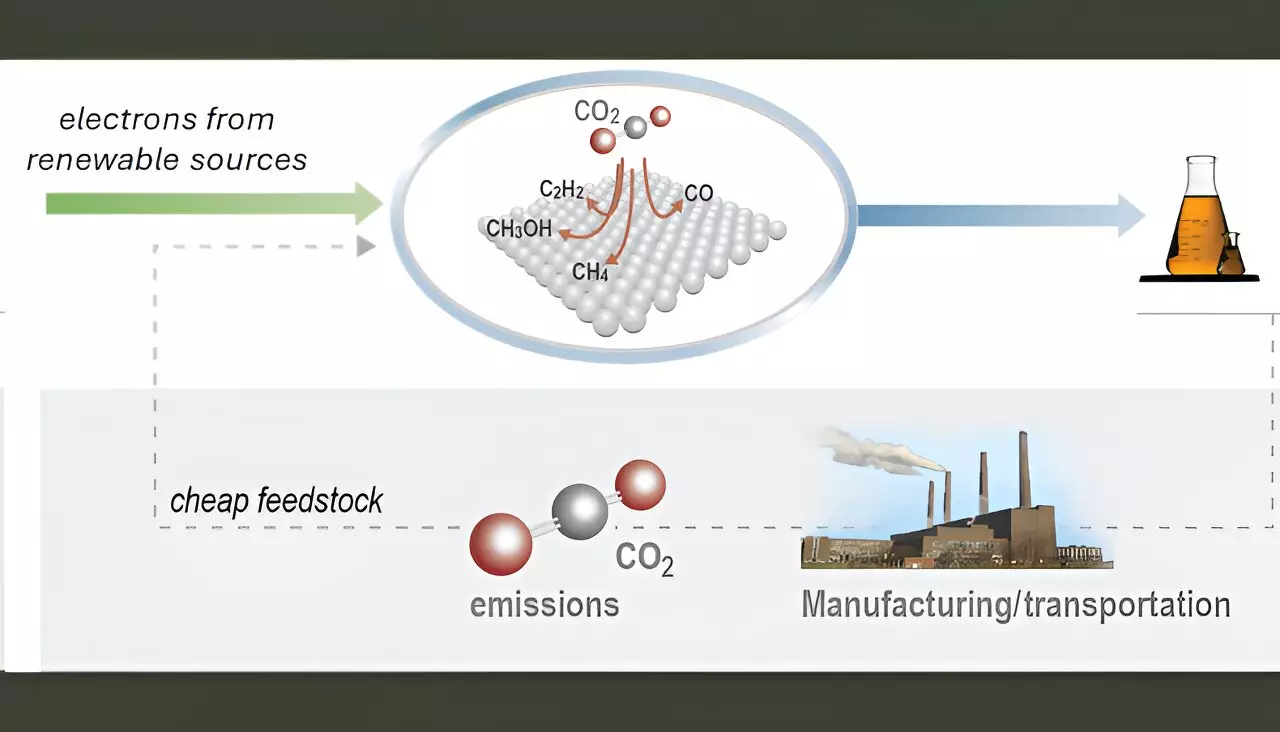
The ever-increasing concern over climate change and the role of greenhouse gases like carbon dioxide (CO2) has driven research into innovative technologies that could alleviate environmental issues while satisfying industrial demands. One promising avenue in this field is the electrochemical conversion of CO2, particularly when integrated with renewable energy sources such as solar and wind power. The goal is to produce valuable chemicals and fuels that can replace fossil-derived counterparts, thus addressing both energy sustainability and carbon emissions.
The transformation of CO2 into high-demand chemicals such as ethylene, ethanol, and acetic acid represents a dual benefit: reducing atmospheric CO2 levels and supplying essential feedstocks for the chemical industry. These products are crucial not just for chemical manufacturing but also for various transportation fuels. However, while theoretical frameworks and initial designs for efficient electrolyzers—devices that facilitate the chemical conversion process using electricity—have emerged, practical commercialization faces significant hurdles. Two critical factors remain at the forefront: catalyst stability and selectivity.
Catalysts play a crucial role in determining the efficiency and effectiveness of the electrochemical conversion process. Difficulty arises when measuring catalyst performance because integration methods can significantly influence outcomes. When different electrolyzer configurations or catalyst materials are tested, variability in results often confounds data interpretation. This highlights the importance of isolating catalyst performance from external integration variables, which is currently an active area of research.
Many efforts thus far have centered around copper and its alloys, which have shown promising results in converting CO2 into valuable multi-carbon products. However, the path forward necessitates a sophisticated understanding of where and how to improve these catalysts to enhance both their stability and selectivity in diverse applications.
A group of scientists from Lawrence Livermore National Laboratory (LLNL) has made significant strides in addressing these challenges. They have developed a novel catalyst coating platform utilizing physical vapor deposition (PVD) technology—a method notable for its precision in controlling various attributes of catalysts, including thickness, composition, morphology, and porosity. This platform promises to significantly advance the field by enabling researchers to systematically investigate copper-based dilute alloy catalysts, which have historically been difficult to create and integrate effectively.
Lead scientist Juergen Biener emphasized the importance of their innovation: this scalable catalyst platform allows for tuning composition without altering morphology. Consequently, researchers have the flexibility needed to optimize the catalysts specifically tailored for CO2 conversion.
The collaboration among LLNL researchers, alongside their peers from the University of Delaware, Washington University, and the University of Pennsylvania, has yielded promising results. They explored the unique properties of copper-based dilute alloy catalysts, aiming to foster effective conversion of intermediate carbon monoxide into target multi-carbon products. Their insights underscore the potential of dilute alloys in modifying the energy landscape of CO2 electrolysis, thus enhancing the feasibility of producing cleaner and more efficient feedstocks.
Joel Varley, a key figure in the simulation aspect of this project, emphasized that the newfound capabilities in utilizing dilute alloys could revolutionize how we approach CO2 transformation, unlocking pathways to cleaner industrial processes.
In addition to its refined capabilities for tuning catalyst properties, PVD technology boasts lower waste production and reduced labor efforts compared to traditional electrodeposition techniques. While the initial capital costs may be higher, the potential for operational cost savings and environmental benefits positions PVD as a forward-thinking approach for sustainable chemical manufacturing and transportation systems. The impacts of improved catalysts could ripple through the chemical and transportation industries by making them more sustainable, enabling a greener future.
As research continues and collaboration deepens across institutions and industries, the nexus of renewable energy and CO2 utilization stands to yield transformative solutions to some of the most pressing challenges of our time. Through focused efforts on catalyst optimization and innovative deposition techniques, we could witness a significant leap forward in both environmental sustainability and economic viability.

Leave a Reply