Click chemistry has revolutionized the way synthetic chemists approach molecular assembly. The methodology combines remarkable efficiency with a focus on selectivity and yield, making it a preferred choice for diverse applications such as pharmaceuticals, materials science, and chemical biology. However, traditional routes to synthesizing certain crucial compounds, specifically sulfonyl fluorides, have relied on hazardous reagents that pose significant environmental and safety concerns.
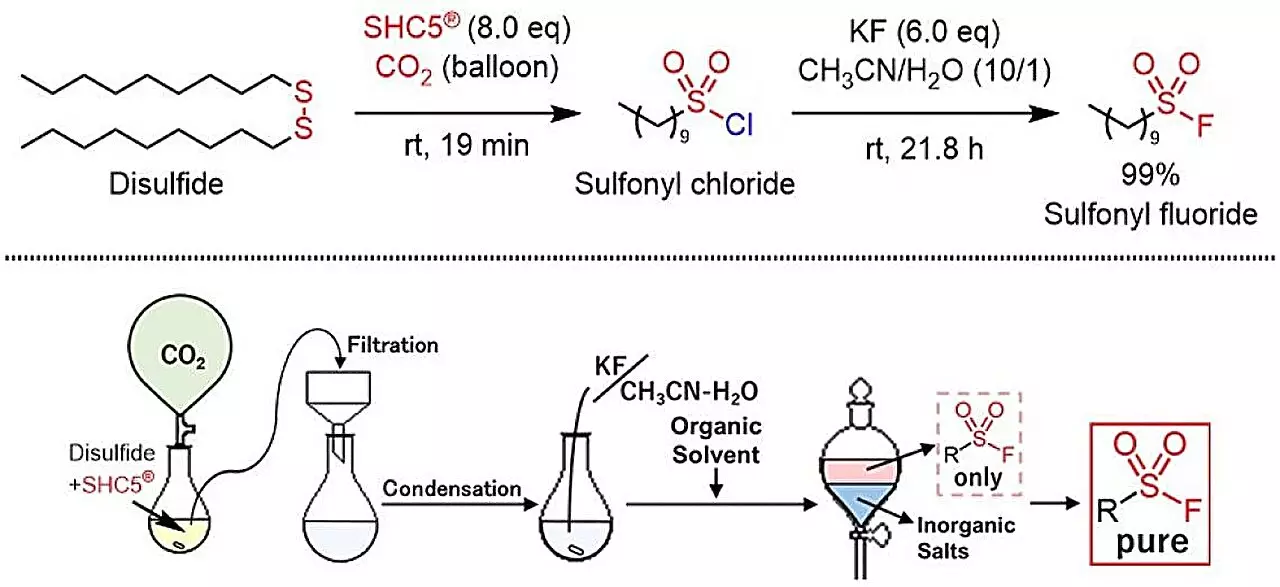
Recent research has unveiled a groundbreaking method for synthesizing sulfonyl fluorides without the need for toxic materials like SO2F2 or KHF2. Published in ACS Sustainable Chemistry & Engineering, this innovative process employs SHC5 and potassium fluoride (KF) to convert thiols and disulfides into sulfonyl fluorides effectively and safely. The significance of this advance cannot be understated; by producing only sodium chloride and potassium chloride as by-products, this method presents a stark alternative to previous techniques that generated harmful emissions.
What distinguishes this newly developed synthesis method is its eco-friendly nature. As the global scientific community increasingly recognizes the importance of sustainable methodologies, the implications of using SHC5 and KF are vast. This method not only simplifies the reaction conditions but also reduces hazards associated with chemical handling. By providing a straightforward and cost-effective pathway, it encourages wider adoption in both research and industrial contexts.
Dynamics of the Reaction Process
The reaction itself has been shown to accommodate various molecular structures, enabling the synthesis of sulfonyl fluorides that include aromatic, aliphatic, and heterocyclic groups. The flexibility of this synthetic protocol allows chemists to tailor the compounds created to meet specific needs, enhancing the utility of sulfonyl fluorides in future applications. This versatility positions the method as a potential cornerstone in industrial chemistry.
Contributions to Sustainability
The study advocates for a broader vision of synthetic chemistry that aligns with global sustainability goals. Authors Masayuki Kirihara, Shinobu Takizawa, and Mohamed S. H. Salem emphasize the pressing need to develop synthetic protocols that minimize their environmental footprint. As the scientific community pushes towards adhering to Sustainable Development Goals (SDGs), processes such as the one introduced in this research pave the way for responsible chemical manufacturing.
The advancements in the synthesis of sulfonyl fluorides through non-toxic and efficient methods signal a crucial development in click chemistry. By prioritizing environmental sustainability while expanding the functional capabilities of chemical synthesis, this research exemplifies the future of organic chemistry. As the method gains traction, it enhances the role of chemists in contributing to a more sustainable world, proving that progress and responsibility can walk hand in hand in the realm of science.

Leave a Reply