Cancer remains one of the most challenging medical conditions, often requiring innovative strategies for effective treatment. A significant aspect of combating this disease lies in halting the proliferation of cancer cells. This involves an intricate understanding of the proteins that these cells depend on for their survival. Recent advancements in protein profiling—an essential tool for cancer research—allow scientists to identify and target these proteins. However, past methodologies faced limitations, often resulting in missed opportunities to discover vital protein targets that could pinpoint future therapeutic interventions.
At the forefront of this quest for better cancer therapies is a collaborative effort by chemists at Scripps Research, who have pioneered a novel dual-method approach to protein analysis. Their work, which was published in **Nature Chemistry**, uncovered over 300 small molecule-reactive cancer proteins along with their specific binding sites. This comprehensive mapping opens avenues for developing more precise cancer treatments by disrupting the interactions between these proteins and their respective chemical compounds, known as small molecules.
Co-senior author Dr. Benjamin Cravatt emphasized the significance of merging two distinct activity-based protein profiling (ABPP) methods. This combination not only allowed for a broader overview of protein interactions with chemical compounds but also provided detailed insights into the exact locations of these interactions. By capturing protein activity on a global scale, the researchers have laid a robust foundation for targeted cancer therapies.
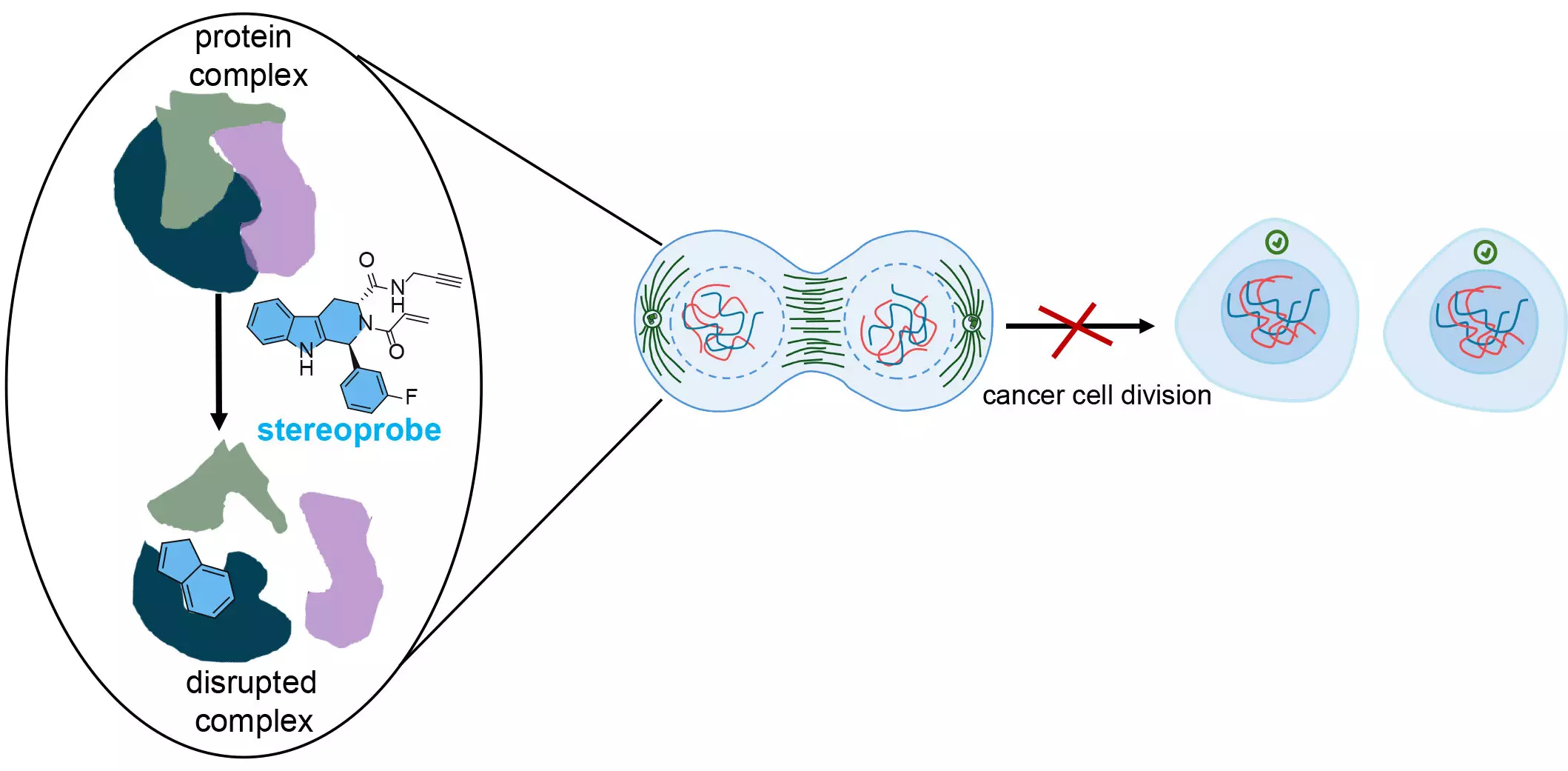
Central to the research is the innovative application of stereoprobes—chemical compounds designed for selective and irreversible binding to target proteins. The team meticulously constructed these stereoprobes to incorporate unique chemical features often overlooked in conventional drug discovery processes. Co-senior author Dr. Bruno Melillo highlighted that this intentional design strategy could yield insights that not only enhance our understanding of protein functions but also lead to breakthroughs in advancing human health.
The stereoprobes specifically target the amino acid cysteine, a prominent feature in many proteins, including those implicated in cancer. Cysteine’s role in maintaining structural bonds within proteins makes it an attractive target for therapeutic intervention. By binding to cysteine, these compounds can disrupt essential molecular interactions, thereby hindering cancer cell growth. First author Evert Njomen elaborated on this concept, describing the ability of these stereoprobes to identify and attach to critical locations within proteins, much like finding the right piece in a puzzle.
To achieve a deeper understanding of these interactions, the team employed a technique called protein-directed ABPP, which unveiled the specific proteins interacting with the stereoprobe library. However, the researchers sought to refine their observations further. Implementing cysteine-directed ABPP enabled them to zoom in on the specific locations of the stereoprobe bindings, offering a meticulous view of protein reactivity.
This dual-method approach revealed significant insights: it not only identified numerous protein targets but also exposed a notable discrepancy in data quality when relying on a singular methodology. This revelation emphasizes the limitations often associated with traditional techniques, reinforcing the necessity for more comprehensive analytical frameworks in cancer research.
The implications of this research extend beyond immediate cancer applications. By honing in on critical protein regions essential for cancer cell survival, the Scripps team hopes to pave the way for developing targeted therapies aimed at inhibiting cell division. Njomen mentioned the potential for these targeted stereoprobes to induce a state within cancer cells that renders them identifiable by the body’s immune system, prompting their elimination.
Moreover, the research team aims to apply their innovative stereoprobe libraries to explore protein involvement in a range of diseases, including inflammatory disorders. The ability to adapt and extend this methodology could enhance our understanding of various pathologies and facilitate the development of targeted therapies beyond cancer.
The groundbreaking findings from the Scripps Research team underscore the importance of comprehensive protein profiling in the war against cancer. Their innovative dual-pronged approach reveals critical interactions between proteins and small molecules, providing a superior pathway for targeted drug development. As research continues, there lies an optimistic potential for these insights to foster novel cancer treatments that improve patient outcomes and revolutionize the treatment landscape for various diseases. The journey of understanding protein functionality and its implication in health continues to be a promising frontier in biomedical research.

Leave a Reply