In a groundbreaking study conducted by scientists from King’s College London in collaboration with Imperial College London, a significant advancement in understanding the enzyme Acetyl-CoA Synthase (ACS) has been made. This research not only sheds light on the functionalities of this crucial enzyme but also presents a potential solution to capturing CO2 from the atmosphere in the ongoing battle against climate change.
Recreating the Active Site of Acetyl-CoA Synthase
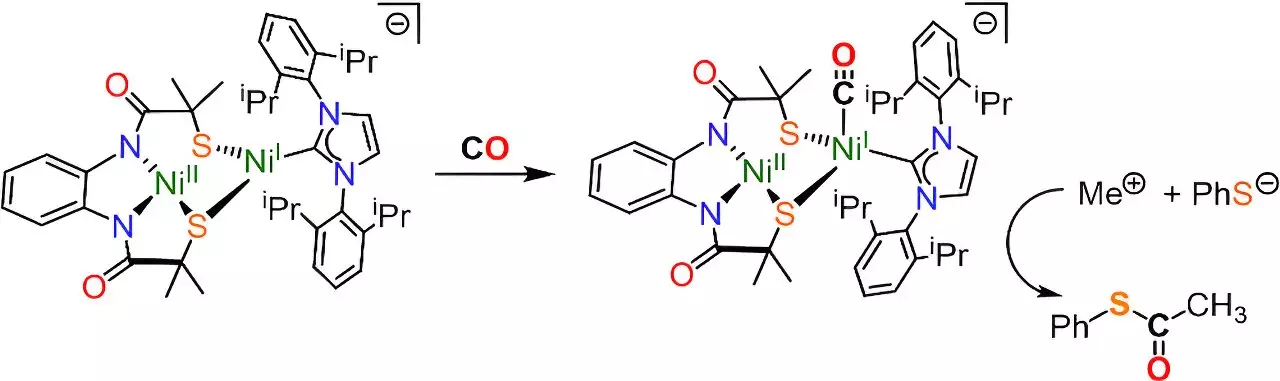
Led by Dr. Rebecca Musgrave and Dr. Daniel Wilson, the team successfully replicated the active site of ACS, where essential chemical reactions take place. ACS plays a vital role in converting CO2 into acetyl coenzyme-A, a fundamental molecule in living organisms. This breakthrough research, published in the Journal of the American Chemical Society, has paved the way for a better understanding of ACS and its significance in the acetic acid cycle or Krebs Cycle.
Importance of Enzymes in Nature
Enzymes are crucial proteins that act as biological catalysts, expediting chemical reactions in various biological processes. Over billions of years, these chemical pathways have evolved into intricate systems that are challenging to study and replicate in a laboratory setting. As such, scientists often create models of enzymes’ active sites to comprehend their mechanisms better.
Previous attempts at replicating the active site of the ACS enzyme fell short in accurately mimicking the shape and electronic environment required for carbon capture. Dr. Daniel Wilson emphasized the longstanding challenge of deciphering the mechanism through which ACS produces acetyl coenzyme-A. However, the team’s new model, featuring a molecular cluster with two nickel atoms, demonstrated remarkable similarities to the ACS enzyme’s active site.
Experimental Techniques and Findings
Utilizing Electron Paramagnetic Spectroscopy, the researchers were able to study the synthesis steps involved in capturing atmospheric carbon and transforming it into acetyl coenzyme-A. This technique provided valuable insights into the workings of the ACS enzyme and other enzymes related to carbon fixation and capture. Dr. Rebecca Musgrave highlighted the potential applications of this research in designing man-made catalysts for industrial purposes, such as capturing CO2 from the atmosphere for producing carbon-based chemicals like biofuels and pharmaceuticals.
The findings of this study hold promise for various fields, including enzyme spectroscopy, where researchers can leverage the new model to further their studies on enzyme functionalities. Dr. Musgrave underscored the efficiency and speed at which enzymes catalyze transformations in nature, making them challenging to replicate artificially. Nonetheless, this research opens avenues for developing innovative solutions for carbon capture and utilization in diverse industrial sectors.
The recent breakthrough in recreating the active site of the ACS enzyme marks a significant milestone in enzyme research for carbon capture. By delving deeper into the mechanisms of enzymes involved in carbon fixation, scientists are paving the way for sustainable solutions to combat climate change. The potential applications of this research extend to areas beyond environmental science, offering new possibilities for industrial innovation and biochemical advancements.

Leave a Reply