Lung diseases rank among the leading causes of death globally, impacting millions each year. Conditions such as chronic obstructive pulmonary disease (COPD) and cystic fibrosis are particularly significant contributors to morbidity and mortality. Despite the dire need for advanced treatments, options remain severely limited, primarily because many medications only manage symptoms without addressing underlying pathologies. While lung transplants can provide relief, the stark reality is the acute shortage of donor organs, leaving many patients without any viable options for long-term recovery. This creates a pressing demand for innovative solutions in the realm of pulmonary healthcare.
Traditionally, researchers have turned to animal models, particularly rodents, to study lung diseases and trial new drugs. However, these models often fail to capture the full complexity of human respiratory conditions, which can lead to misleading results regarding the efficacy and safety of experimental treatments. The quest for more accurate models has prompted bioengineers to explore alternatives, including the creation of functional lung tissues in laboratory settings. Yet, one of the foremost challenges in 3D bioprinting such tissues lies in the development of effective bioinks that can support cell growth and mimic the natural environment of lung tissue.
Recent research published in ACS Applied Bio Materials represents a significant advancement in the effort to bioengineer lung tissues. A team led by Ashok Raichur has manufactured a novel mucus-based bioink utilizing mucin, an important component of mucus that plays a crucial role in various biological functions. The researchers explored mucin’s potential, revealing its unique molecular structure that bears similarity to epidermal growth factor (EGF), a protein essential for encouraging cell attachment and proliferation. This innovative approach not only expands the utility of bioprinting but also demonstrates a remarkable step toward creating viable lung tissue models.
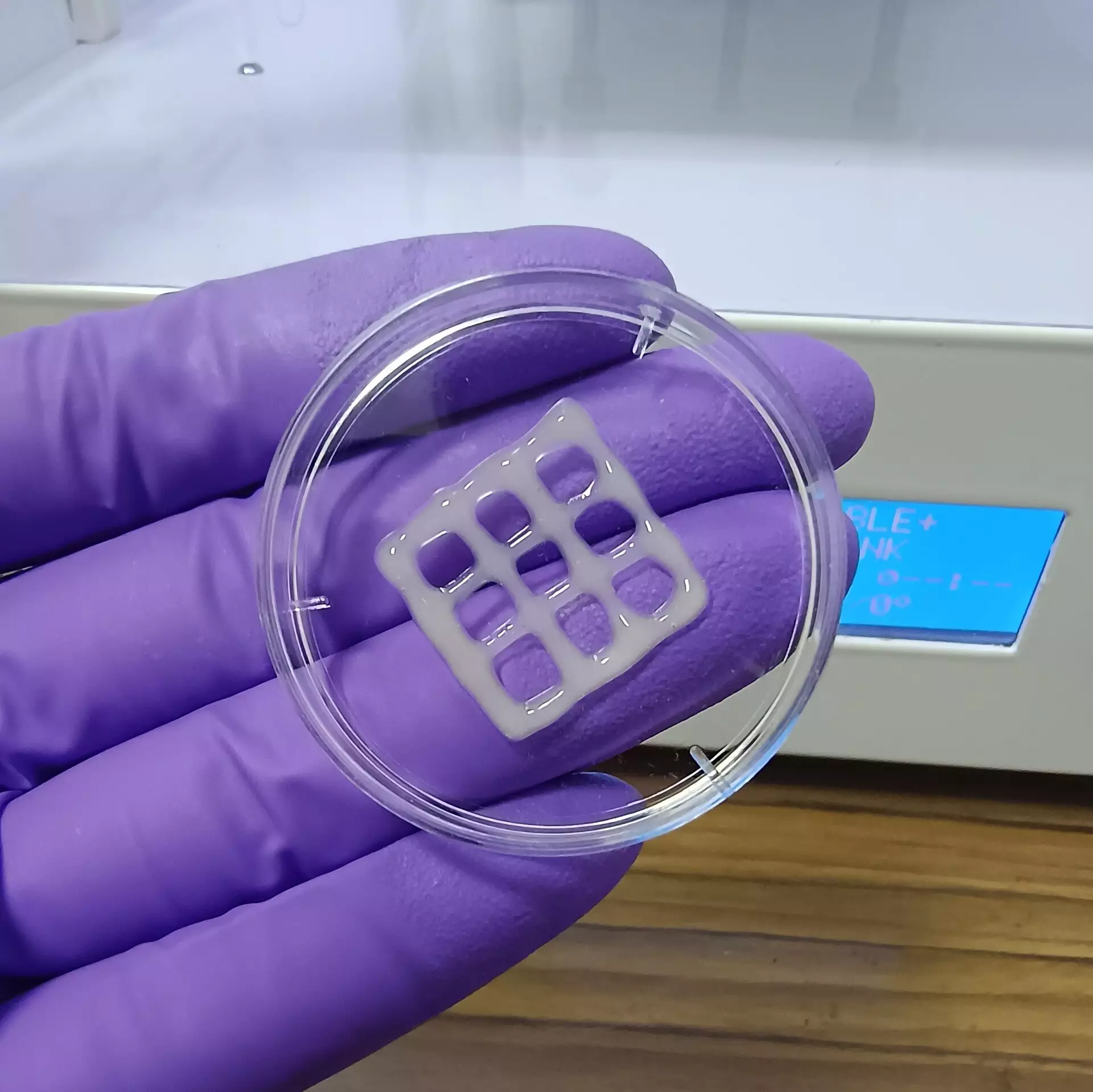
To produce this bioink, Raichur and his team reacted mucin with methacrylic anhydride, resulting in a compound called methacrylated mucin (MuMA). By mixing MuMA with lung cells and adding hyaluronic acid—another natural polymer known for its role in promoting cell adhesion and growth—they enhanced the viscosity and overall functionality of the bioink. The newly formulated bioink was then subjected to 3D printing, forming intricate patterns, including grid designs that provide a suitable scaffold for lung cells. Using blue light to crosslink the MuMA molecules, the team successfully stabilized these structures into porous gels, which exhibited excellent water absorption properties crucial for cell nutrition and survival.
Implications for Future Research
The team’s findings suggest that the interconnected porous structure of the printed gel not only supports the survival of lung cells but also promotes nutrient diffusion and overall tissue growth. Critical results from the experiments highlighted that the printed constructs were non-toxic and, significantly, displayed a slow biodegradation process under physiological conditions. This characteristic is particularly noteworthy as it paves the way for using these 3D-printed scaffolds as potential implants, whereby engineered tissue can gradually replace the synthetic scaffold with biologically active lung tissue.
The implications of this research are vast, extending beyond mere tissue engineering. The bioink developed could serve as a revolutionary tool for producing highly accurate 3D models of human lungs, invaluable for studying the pathophysiology of lung diseases and accelerating the evaluation of new treatment strategies. As researchers continue to unravel the complexities of lung disorders through these models, the hope is that more effective therapies will emerge, ultimately alleviating the burdens posed by these debilitating conditions.
The innovative approach to creating mucus-based bioink marks a pivotal moment in the realm of lung tissue engineering. With the potential to enhance our understanding of various pulmonary diseases while also paving the way for new therapies, this research could significantly change the landscape of treatment options available for millions worldwide. As scientists build on these discoveries, the future of respiratory health may see a shift from limited, often inadequate treatments to a new era of regenerative medicine that holds more promise and hope for patients suffering from chronic lung diseases.

Leave a Reply