Ammonia is a vital compound not only in agriculture, where it serves as a key fertilizer, but also plays an emergent role in sustainable energy as a zero-carbon fuel. The unique properties of ammonia, including its high energy density, make it an ideal candidate for fueling the future without the adverse greenhouse gas emissions associated with conventional energy sources. Despite its potential, the conventional route for ammonia production, the Haber-Bosch process, is notoriously energy-intensive, accounting for approximately 1.8% of the world’s carbon dioxide emissions. In light of these concerns, researchers have turned to alternative methods for ammonia generation, such as the electrochemical nitrate reduction reaction (eNO3RR), which offers a more sustainable route using nitrate, a common pollutant.
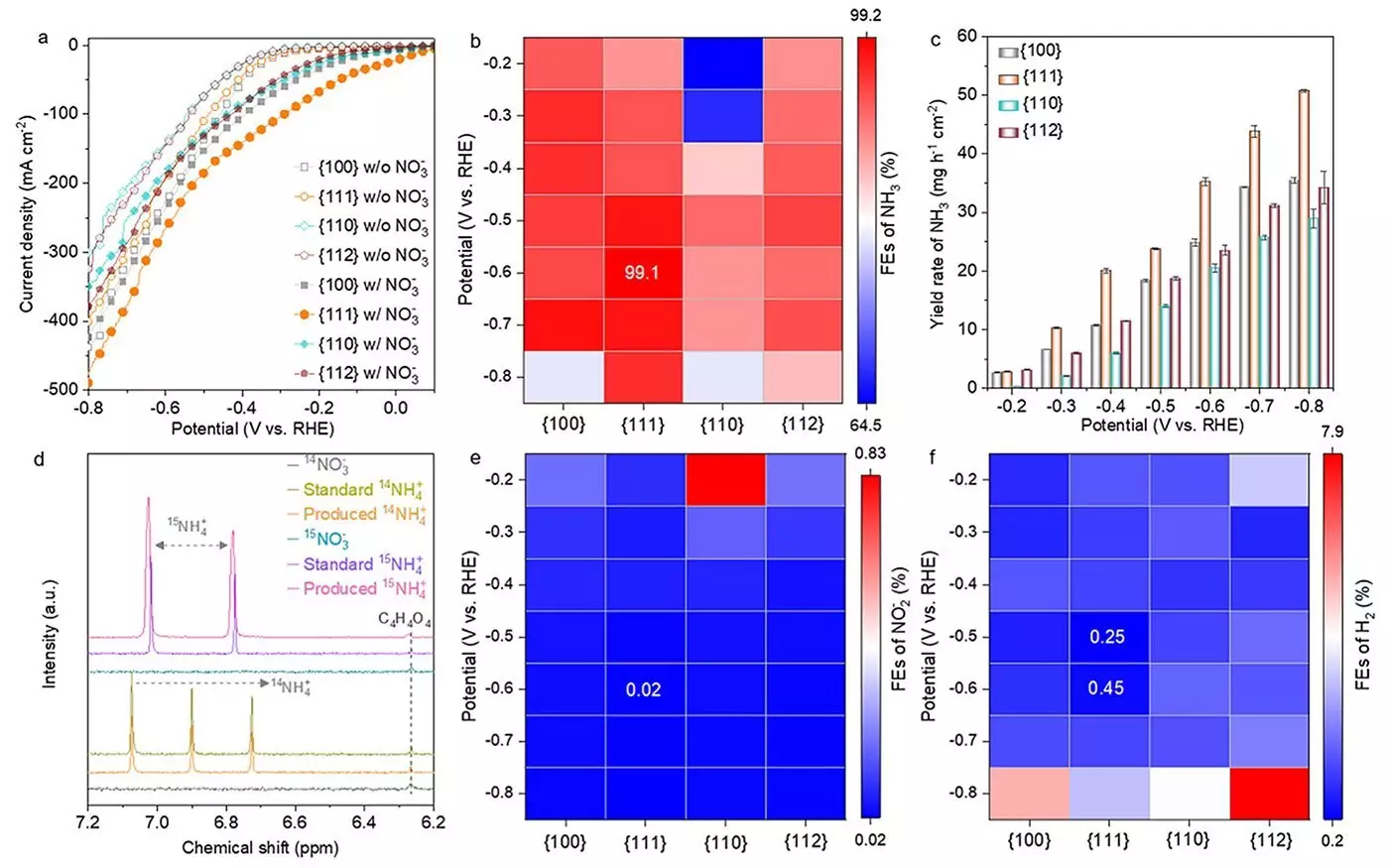
A recent study published in ACS Nano has unveiled exciting advancements in catalyst technology that promise to optimize the eNO3RR process significantly. The research team focused on developing cobalt oxide catalysts (Co3O4) characterized by their affordability and remarkable catalytic activity. The study explored the performance of various nanostructures of Co3O4, differentiating based on their crystallographic facets: {100}, {111}, {110}, and {112}. This examination aimed to understand how the structural variations among these facets affect the efficiency of ammonia production.
Their findings highlighted that the {111} facet surpassed other configurations in terms of performance, achieving an astonishing ammonia Faradaic efficiency of 99.1% and a yield rate of 35.2 mg h-1 cm-2. According to Dr. Heng Liu, a key figure in the study and an Assistant Professor at the WPI-AIMR, the exceptional performance of the {111} facet can be attributed to its unique ability to generate oxygen vacancies and Co(OH)2 during the reaction process. These findings elucidate the underlying physical chemistry that makes specific structural arrangements more effective than others.
One of the more intriguing revelations from the research was the transformation that the catalyst undergoes during the reaction. Initially, the Co3O4 structure evolves from its original form, transitioning towards a complex hybrid of Co3O4-x-Ov/Co(OH)2 before ultimately stabilizing as Co(OH)2. This dynamic change stayed particularly pronounced on the {111} facet, underpinning its superior catalytic properties. Professor Hao Li, who led the study, emphasized the significance of these structural transformations, stating that understanding these changes is crucial for refining future catalyst designs to maximize their effectiveness.
The implications of these developments are far-reaching. By further controlling the structural and chemical transformations of catalysts, scientists can design even more effective materials for ammonia production. This research represents a fundamental shift toward more efficient utilization of resources by transforming nitrate waste into a valuable societal asset.
Beyond the immediate challenges faced in ammonia production, the advancement of efficient catalysts also contributes to broader efforts in environmental sustainability. The eNO3RR presents an opportunity not only to generate ammonia sustainably but also to remediate nitrate pollution that adversely affects ecosystems. As these catalysts are perfected, they will play an integral role in advancing technologies aimed at achieving carbon neutrality by the mid-21st century.
The research underscores a critical pathway for future endeavors in electrochemical processes, with the potential to reshape current industrial practices that contribute to environmental degradation. The ongoing aim will be to refine catalyst behaviors, focusing on stability and selectivity to create methods that can be scaled for real-world applications.
The breakthrough in understanding the Co3O4 catalysts marks a pivotal point in the quest for sustainable ammonia production. As researchers continue to unravel the complexities of these materials, the journey towards more eco-friendly industrial practices is not only plausible but also increasingly urgent. The path forged by this study serves as a blueprint for future innovations that align energy practices with sustainability goals.

Leave a Reply