In a world that’s increasingly driven by innovation and technological advancement, the discovery of new properties within common materials can herald significant changes across several industries. Such is the case with gallium—a remarkable metal regarded for its low melting point and unique atomic characteristics. Recent research conducted by a team of scientists from the University of Auckland surfaced findings that challenge existing theories about this enigmatic element, potentially altering our understanding of its role in various applications, especially in the fields of nanotechnology and materials science.
Gallium, which was first isolated by French chemist Paul-Émile Lecoq de Boisbaudran back in 1875, combines peculiar physical properties, such as the ability to melt in warm water, with extensive utility, particularly in semiconductor technology. Its unique molecular configuration, specifically its tendency to exist as ‘dimers’ (pairs of atoms), sets it apart from traditional metals, leading to fascinating explorations into its behavior at both solid and liquid states.
Shifting Paradigms: Covalent Bonds and Atomic Behavior
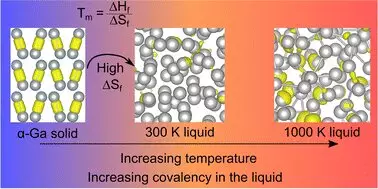
The groundbreaking insights stemming from recent studies reveal that gallium behaves counterintuitively compared to typical metals. Usually, the covalent bonds formed by shared electrons disappear when transitioning to a liquid state. However, researchers found that in gallium, these bonds unexpectedly re-emerge at elevated temperatures—a revelation that contradicts a long-held assumption about its melting behavior.
Professor Nicola Gaston—a leading researcher in this field—has aptly noted that this fundamental shift in understanding necessitates a broader re-evaluation of gallium’s low melting point. The implications are profound, as this newfound knowledge redefines our grasp of atomic interactions within gallium, emphasizing the role of entropy—the degree of disorder within a system. By allowing gallium’s atomic structure to gain freedom upon melting, we can begin to understand the intricate dance of forces at play in this unusual metal.
The Path to Discovery: Revisiting Historical Data
This dizzying leap in knowledge stems from the diligent work of Dr. Steph Lambie, who meticulously reviewed decades of scientific literature and experimental temperature data. As a former Ph.D. student, Lambie’s efforts illuminated previously overlooked details within a wealth of information. The research team, which also included Dr. Krista Steenbergen, underscored the importance of collaboration across institutions to piece together the threads of a complex yet essential material’s behavior.
This careful synthesis of past research highlights a larger trend in scientific inquiry: the willingness to revisit and question established knowledge. It’s an acknowledgment that our understanding is ever-evolving, and fresh insights can come from scrutinizing the previous assumptions that have long been accepted as truth.
Broader Implications: From Nanotechnology to Mars Exploration
Beyond the academic confines of chemistry, the newfound understanding of gallium has real-world implications that could change industries. The metal’s ability to act as a catalyst in liquid metal forms opens up extraordinary opportunities for creating advanced materials that can self-assemble into desired structures—a core concept in nanotechnology.
Moreover, the utility of gallium extends even to astrobiology, as scientists aim to discover traces of ancient microbial life on Mars. Here, gallium might serve as a valuable chemical “fingerprint” helping to trace back the planet’s biological history. This potential application adds an unexpected twist to gallium’s narrative, elevating a scientific inquiry about a metal originally considered mundane to the forefront of extraterrestrial studies.
A Brighter Future for Gallium-Based Technologies
The surge of interest in gallium also reflects broader trends in material science. As we seek sustainable and efficient alternatives to traditional technologies, gallium’s inclusion in various sectors—from aerospace to solar energy—continues to underscore the necessity of integrating novel materials into our technological arsenal.
As we advance into the future, the revelations about gallium will undoubtedly serve as a catalyst—quite literally—for innovations that we have yet to envisage. By continuing to explore the complexities of gallium and materials alike, researchers are not just reimagining what these elements can do; they are charting pathways toward revolutionary applications that could redefine our world.
In essence, gallium’s enigmatic behavior acts as a reminder that science is a continually unfolding tapestry, rich with discoveries waiting just beneath the surface for those curious enough to explore.

Leave a Reply