Ice exists in a world where it is perpetually surrounded by liquid water, creating a mysterious interface that has puzzled scientists for generations. The interaction between ice and liquid is not just a physical phenomenon; it’s fundamental to various environmental and biological processes. From the slips we take on icy sidewalks to the formation of snowflakes, these everyday experiences are influenced by the intricate relationships at the ice-water interface. Understanding this relationship can bring new insights to fields as diverse as meteorology, climate science, and material engineering.
A groundbreaking study conducted by a team from Kobe University and the Institute for Molecular Science has made significant strides in unraveling this complex interaction. Utilizing antifreeze and a state-of-the-art refrigerated microscope, they have succeeded for the first time in directly observing the precise shape of ice at its junction with liquid water.
A Novel Scientific Approach
One of the most striking challenges that scientists have faced in studying ice is its elusive nature—ice and water rapidly shift between states, making it difficult to stabilize observations at the interface without external interference. In a remarkable twist of ingenuity, principal researcher Onishi Hiroshi conceived a method to immerse ice in antifreeze that remains colder than freezing point, cleverly allowing the ice to maintain its solid form while observing its characteristics directly.
This clever workaround leads to a cleaner pathway that allows for clearer observations, yet success was not easily obtained. The research team encountered numerous roadblocks in trying to obtain vital measurements. As Hiroshi explains, “We had to cool the entire microscope system in a cooling box, and it took some ingenuity to ensure that the atomic force microscope, a precision measuring instrument, could operate stably at sub-zero temperatures.” This inventive spirit showcases the resilience of scientific inquiry—through trial and error, they achieved what was once thought to be impossible.
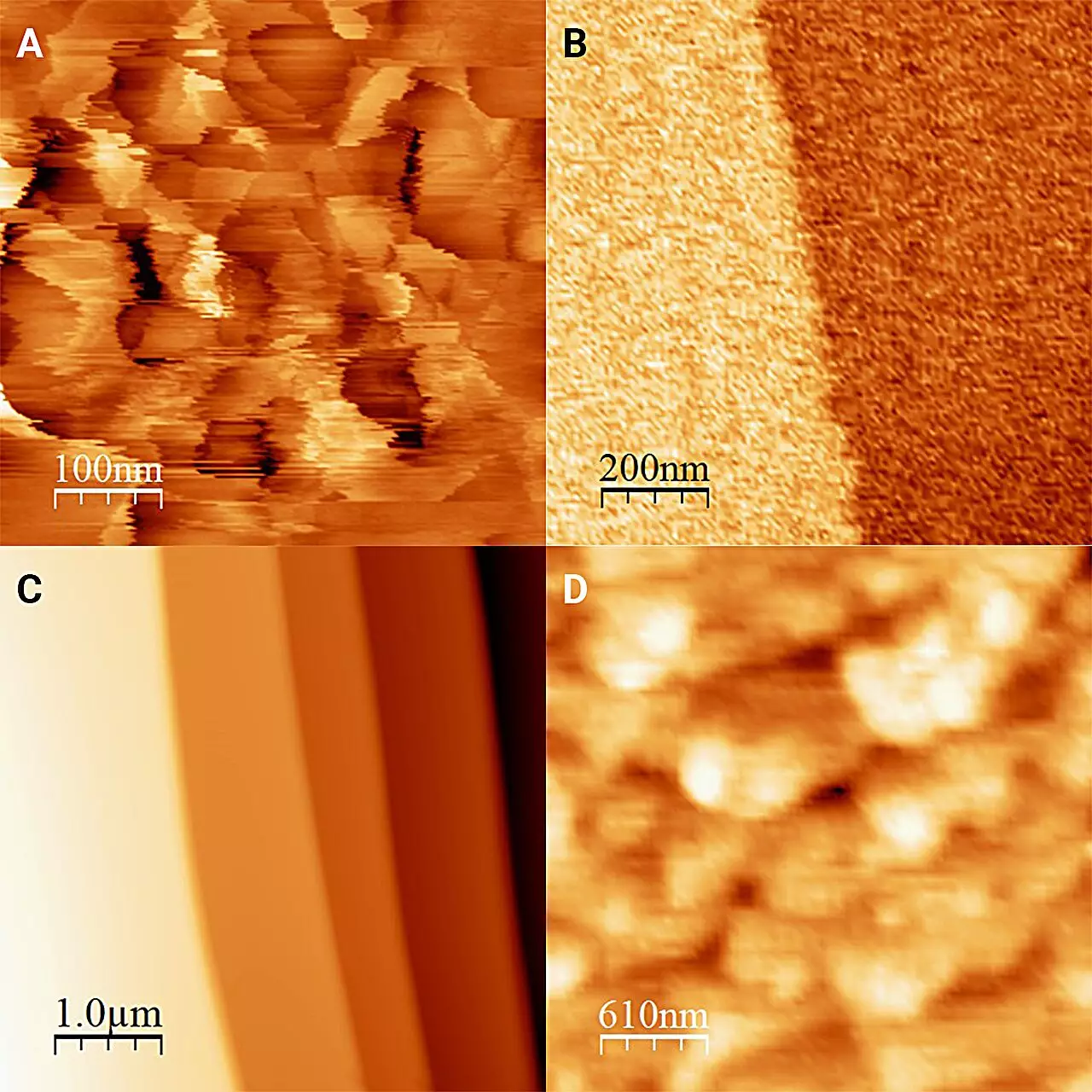
Revolutionary Findings and Implications
Published in The Journal of Chemical Physics, the researchers unveiled some shocking results. Whereas traditional ice forms numerous “frost pillars” around 20 nanometers in height when left in the open air, the ice subjected to antifreeze appeared remarkably smooth, with an almost perfectly flat surface interrupted by occasional molecular-level steps. This change was attributed to the partial dissolution and recrystallization of the ice surface within the antifreeze medium, indicating that the environment around ice significantly influences its structural properties.
Moreover, the team extended their exploration beyond a single liquid, experimenting with various types of alcohol such as 1-octanol. Astonishingly, they observed variations in the ice surface across different liquids, underscoring the dynamic nature of the ice-liquids interface. This finding reinforces the principle that direct measurement is invaluable when trying to unravel the complexities of material interactions.
Another noteworthy discovery pertained to the “hardness” of the ice surface when submerged in 1-octanol. Contrary to previous estimations that determined the ice to be softer under typical conditions, the researchers found that the ice was more rigid than previously believed. Such insights can profoundly impact our understanding of how ice behaves in natural environments and its role in climate change dynamics.
Future Directions and Innovations
Inspired by their findings, Hiroshi and his team have their sights set on pushing boundaries further. They hope to enhance their measurement techniques to capture images at the resolution of individual water molecules, a level of detail that could transform our understanding of molecular interactions at the ice-liquids boundary. Exploring measurement methodologies beyond atomic force microscopy could also usher in a new era of research into ice dynamics and its broader implications.
This innovative study is a testament to the power of creativity in science. The breakthroughs achieved by the Kobe University team not only deepen our understanding of a fundamental natural phenomenon but also open doors to future research that could address pressing environmental questions, particularly in a world increasingly affected by climate change. Understanding ice may seem remote for many, but in truth, it is deeply intertwined with our everyday life and vital for various scientific disciplines.

Leave a Reply