Z-alkenes, characterized by the presence of a double bond and substituents on the same side, play a pivotal role in various organic compounds. Their unique structural attributes make them essential in both chemistry and biology. Often, these compounds can be elusive, resisting conventional preparation methods due to their complex thermodynamic properties. However, the landscape of Z-alkene synthesis is changing, thanks to innovative approaches like photoisomerization. This process, which utilizes light to convert E-alkenes to Z-alkenes, has the potential to revolutionize not just research methodologies but also applicable techniques across fields, including organic and medicinal chemistry.
Photoisomerization involves altering the arrangement of a molecule through light absorption—a process that can yield significant production of Z-alkenes. This transformation is especially crucial because many traditional thermodynamic methods fall short of achieving satisfactory yields for Z-alkenes. Research has shown that photoisomerization can bypass these challenges, unlocking pathways that were previously inaccessible. Various studies have demonstrated the utility of different photoisomerization techniques, but a recent breakthrough from a team in Japan has brought a fresh perspective to this evolving field.
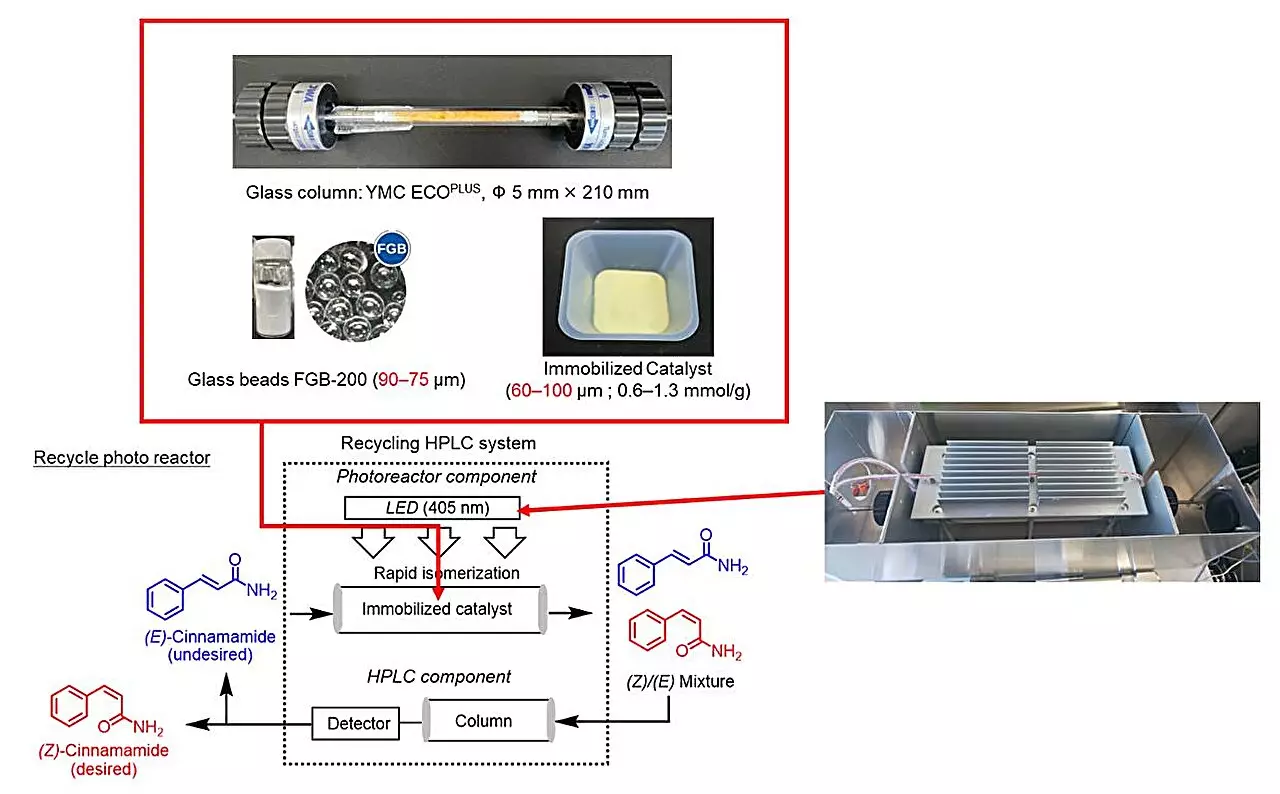
Led by Professor Hideyo Takahashi from Tokyo University of Science, a dedicated team has ventured into unexplored territory with their new research published in *The Journal of Organic Chemistry*. This innovative study focused on the photoisomerization of E-cinnamamides into their Z-cinnamate counterparts, employing a novel recycling photoreactor coupled with high-performance liquid chromatography (HPLC). This approach represents a significant leap forward in the recycling and efficiency of chemical reactions, aligning with current demands for sustainable practices in organic chemistry.
A major hurdle in the successful synthesis of Z-alkenes via photoisomerization is the selection of an effective photosensitizer. This vital component serves as a catalyst that speeds up the reaction by absorbing light energy and transferring it to the reactant. Overcoming this challenge, the research team meticulously screened various commercially available photosensitizers, ultimately identifying thioxanthone with functional amide groups as the optimal choice. Their strategic immobilization on modified silica gel not only prevented leakage but also significantly enhanced catalytic activity—an impressive feat considering that solid-phase reactions typically lag behind their liquid counterparts. The study revealed that careful consideration of functional groups in catalysts can lead to enhanced efficiency, ushering in a new era for reactions involving Z-alkenes.
One of the standout features of this innovative approach is the closed-loop recycling photoreactor system. Initially designed for deracemization, this unique system was adapted for the conversion of E-cinnamamides to Z-cinnamamides, revolutionizing how chemical reactions can be conducted. The integration of HPLC allows for the continuous recycling of samples, ultimately promoting greater efficiency and sustainability in chemical manufacturing. As Professor Takahashi notes, such a system presents a compelling alternative to traditional methods. Its closed-loop nature not only minimizes waste but aligns with the growing demand for environmentally friendly solutions in chemical production.
As global initiatives increasingly prioritize sustainability, the resurgence of Z-alkenes facilitated by sophisticated methods like the one developed by Takahashi and colleagues could not be more timely. With pharmaceuticals and a multitude of organic compounds relying on these structures, the implications of their research extend far beyond the lab. This new framework could redefine synthesis practices in organic chemistry, making them more efficient, cost-effective, and less harmful to the environment. The continued exploration of alternative methods, such as photochemical processes, holds promise for the discovery and production of new compounds that could significantly advance medicinal chemistry.
This innovative study not only underscores the potential of Z-alkenes but also promotes a paradigm shift towards sustainable chemistry that could lay the groundwork for the development of more eco-friendly pharmaceutical products. In doing so, it paves the way for a future where scientific advancement harmonizes with environmental responsibility.

Leave a Reply