In the quest for sustainable energy solutions, hydrogen stands out as a potential frontrunner. Known for its clean-burning properties, hydrogen can be produced in a variety of ways, with electrolytic water splitting being one of the most promising methods. This process involves using electricity to disassociate water molecules into hydrogen and oxygen, and it is inherently tied to technological advancements in photoelectrochemical cells (PECs). These innovative devices utilize sunlight and specialized electrodes to facilitate water splitting efficiently. Recent research indicates that increasing the operational pressure within these cells can significantly enhance their efficiency, offering a pathway toward more effective hydrogen production.
At the heart of PEC technology lies the concept of mimicking nature’s photosynthesis process. While green plants utilize the natural photosystem II to convert solar energy into chemical energy, PEC cells employ artificial, inorganic materials as photoelectrodes. These electrodes generate the necessary voltage to drive the electrolytic reaction when exposed to sunlight. Current advancements have led to impressive energy conversion efficiencies nearing 19%. However, these gains are not without challenges. One of the critical issues that arise, particularly at high efficiency levels, is bubble formation during the electrolysis process.
Challenges Posed by Bubble Formation
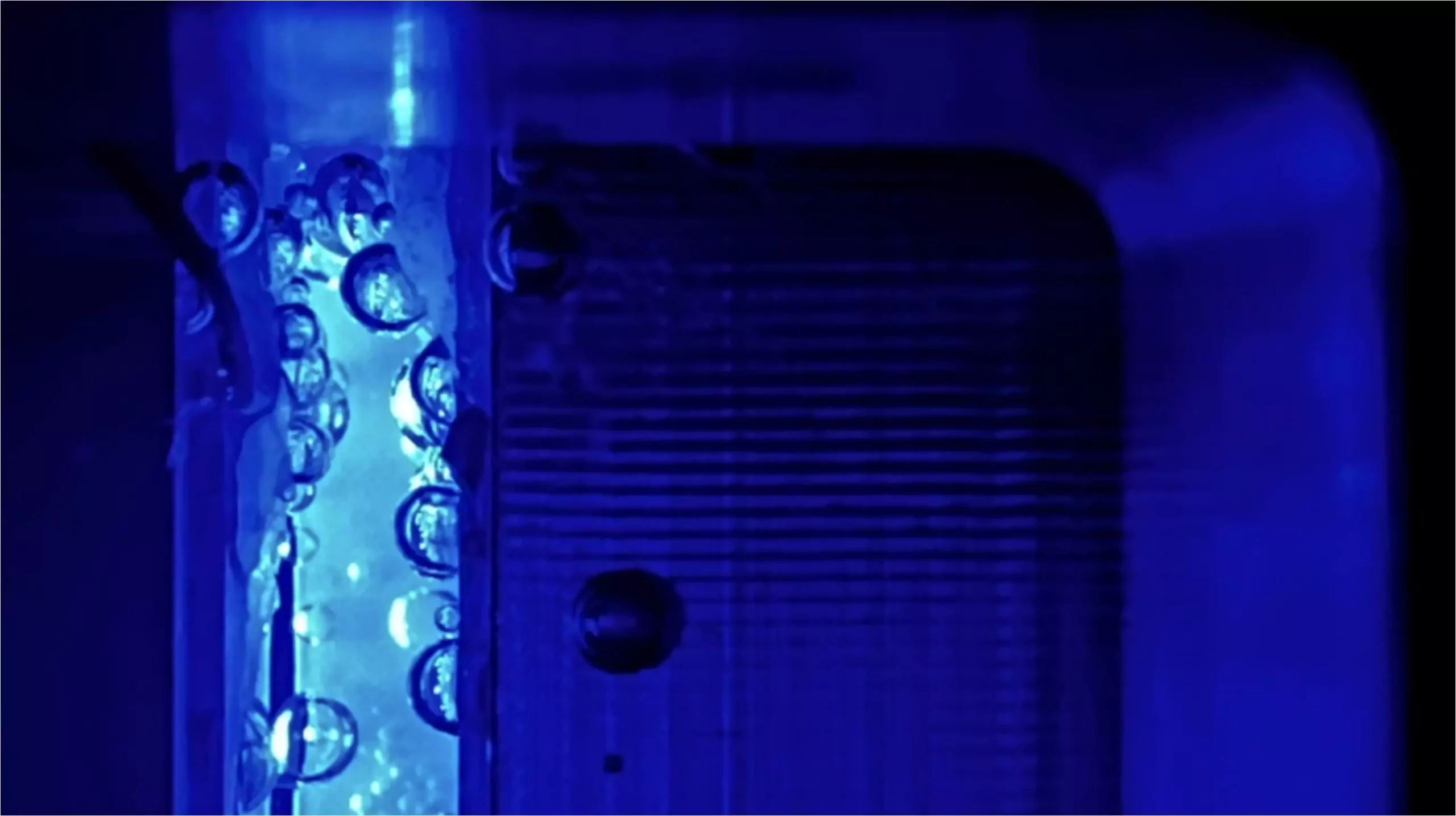
The generation of gas bubbles during water splitting can lead to serious drawbacks for PEC devices. Bubbles can obstruct light, scattering it and thus preventing optimal illumination from reaching the electrodes. Additionally, these bubbles can inhibit electrolyte contact with the electrode surface, leading to what is known as electrochemical deactivation. This interference fundamentally impacts the overall efficiency of the PEC cells, prompting researchers to seek solutions to mitigate these effects. Addressing the size and behavior of bubbles during electrolysis becomes crucial, and one promising pathway involves adjusting the operating pressure within the PEC systems.
Researchers at the Institute for Solar Fuels at Helmholtz Zentrum Berlin (HZB) have embarked on an ambitious study to evaluate the effects of elevated pressure on PEC operations. By pressurizing their systems between 1 and 10 bar, they were able to systematically analyze several parameters affecting electrolysis performance. This experimental setup was complemented by the development of a multiphysics model that simulates the PEC process under various conditions. The insights gleaned from this combined approach are paving the way for advancements in hydrogen production efficiency.
One of the significant findings from this research indicates that operating at an elevated pressure of around 8 bar can reduce total energy losses by half. This adjustment translates to a relative efficiency increase of approximately 5-10%. Such a reduction is remarkable, as it not only alleviates optical scattering losses—allowed by smaller bubble sizes—but also mitigates unwanted product crossover, specifically the transfer of oxygen to the counter electrode. Yet, the researchers note that increasing pressure beyond this optimal level yields no additional benefits, suggesting a sweet spot between 6 and 8 bar for PEC electrolyzers.
Broader Implications for Electrochemical Devices
The implications of these findings extend beyond hydrogen production alone. The multiphysics model developed during this research holds potential for optimizing various electrochemical and photocatalytic systems, offering valuable insights into their operational efficiencies. According to Prof. Dr. Roel van de Krol, the head of the Institute for Solar Fuels, these findings are foundational for future research and development aimed at improving efficiency across a spectrum of renewable energy technologies.
Harnessing the benefits of increased operational pressure in PEC cells emerges as a transformative approach to enhance hydrogen production efficiencies. As research continues in this area, the potential for hydrogen as a clean energy source becomes increasingly viable, ushering in a new era of sustainable energy solutions.

Leave a Reply