The concept of self-organization has long fascinated scientists, particularly in the context of understanding how lifeless matter can spontaneously give rise to complex biological structures. The research led by Professor Anđela Šarić and her team at the Institute of Science and Technology Austria (ISTA) offers exciting insights into one such process: the bacterial cell division. Through a combination of computational modeling and experimental verification, their study, recently published in Nature Physics, sheds light on the peculiar dynamics of a protein called FtsZ that plays a critical role during cell division.
The research revolves around a seemingly paradoxical mechanism termed “dying to align,” where misaligned FtsZ filaments undergo a form of disassembly upon encountering an obstacle. This process aids in the formation of a correctly structured division ring at the cell’s center, essential for the separation of daughter cells during bacterial division. The study raises essential questions about how biological systems manage to organize themselves so efficiently and what principles govern these phenomena.
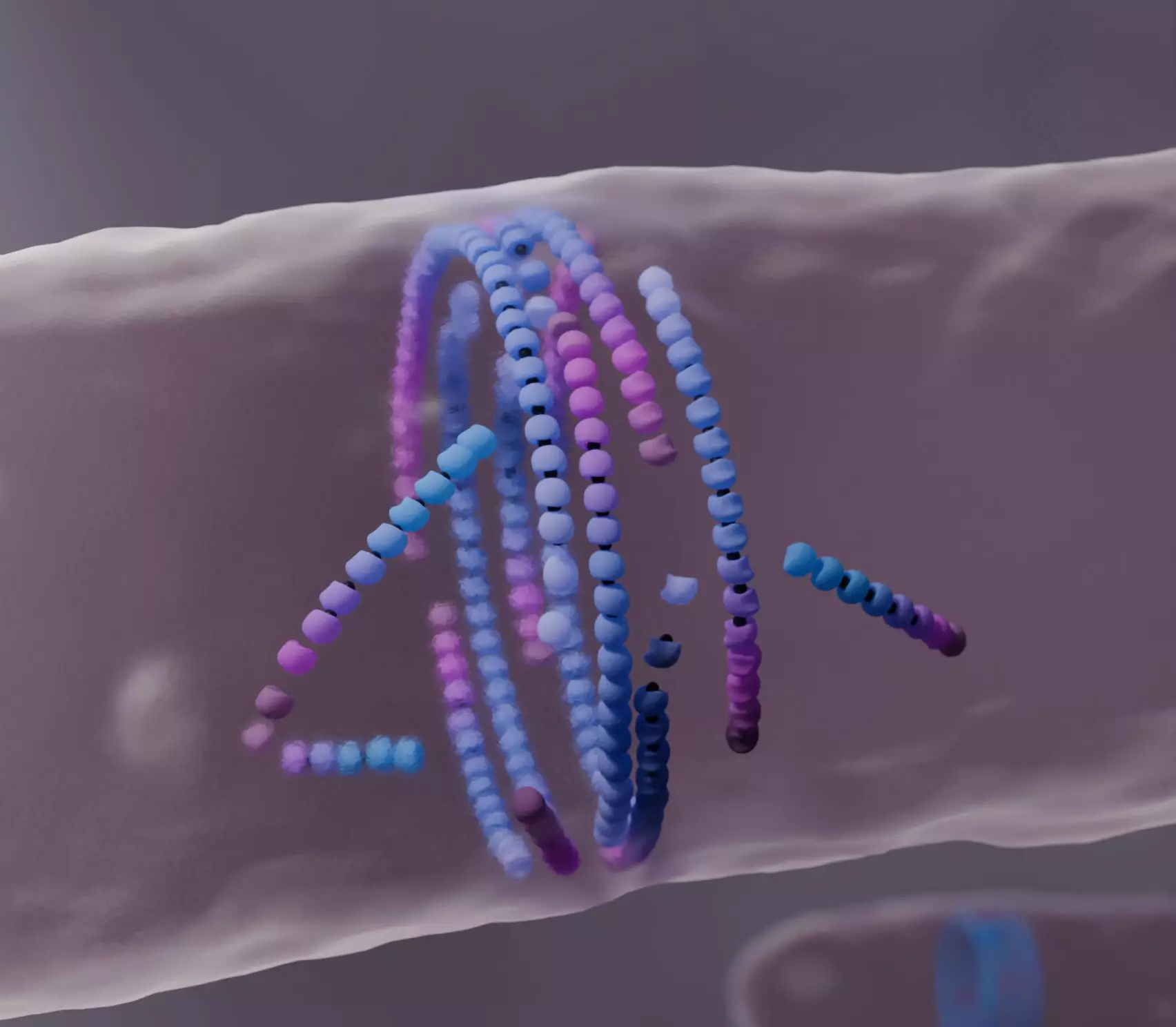
FtsZ is a highly conserved protein found in many bacteria, known for its ability to self-assemble into a ring structure: the bacterial division ring. During the crucial phase of cell division, this filamentous structure serves as a scaffold for building a new cell wall that will ultimately separate the two daughter cells. Interestingly, researchers had yet to fully comprehend how FtsZ assembles itself with such precision despite the constantly shifting dynamics of its molecular subunits.
Utilizing computational models, the Šarić group has sought to unravel this complexity, identifying the key behavior of FtsZ filaments as they “treadmill”—a process defined as the continuous addition and removal of subunits at opposing ends of the filament. While conventional models often portrayed filament growth as a linear and direct force, the Šarić team’s findings demonstrate a more nuanced reality: that misaligned filaments must undergo a sort of “death” before contributing to the orderly assembly of the division ring.
One of the strengths of this research lies in the collaboration between computational scientists and experimental biologists. Through partnerships with researchers such as Séamus Holden from the University of Warwick and Martin Loose from ISTA, the Šarić team’s theoretical predictions were validated through live cell imaging and controlled laboratory experiments. This interdisciplinary synergy illustrates the importance of cross-field alliances in tackling complex scientific challenges and enables a more comprehensive understanding of biological processes.
Absorbing data from experiments observed in live cells, the research highlights how the birth and death of FtsZ filaments correlate directly with the formation of the division ring. The observations made during live-cell imaging resonate perfectly with the hypothetical frameworks presented in the simulations, reaffirming the robustness of their computational approach.
Beyond the immediate biochemical understanding of bacterial cell division, the implications of this research extend to the development of synthetic materials. The principles of self-organization and the adaptive behavior of active matter observed in FtsZ could revolutionize the fields of material science and bioengineering. Specifically, the findings may contribute to the creation of synthetic self-healing materials that mimic the dynamic properties of biological systems.
Šarić expresses an ambitious vision: to explore how the mechanisms underlying living matter can inform the design of non-living materials that exhibit lifelike properties. By leveraging the turnover dynamics of proteins like FtsZ, researchers aspire to forge materials that are not only durable but also capable of self-repair by emulating the efficient processes seen in biological systems such as bacteria.
The Šarić group’s continued exploration into FtsZ and its function in bacterial cell division represents just the beginning of understanding active matter’s potential. Future research will delve deeper into modeling the intricacies of how the division ring aids in constructing the cell wall that partitions the newly formed daughter cells. Each revelation will further illuminate the unique behaviors of biological materials, potentially unlocking new scientific doors.
The groundbreaking work by Šarić and her colleagues illustrates the fascinating dynamism of biological systems, particularly in the context of bacterial division. By analyzing the energetic investments of self-assembling structures like FtsZ, researchers are not only uncovering the secrets of cell biology but also inspiring innovations in synthetic materials that could change our approach to engineering and medicine. As our understanding deepens, the boundaries between the living and non-living continue to blur, promising a future rich with scientific potential.

Leave a Reply