As the global community strives to combat climate change, decarbonizing the energy sector has become an urgent priority. Seawater electrolysis presents a promising avenue to produce hydrogen fuel sustainably, but the process is fraught with challenges. Among the critical obstacles are anode corrosion due to chloride ions, unwanted reactions initiated by chloride oxidation, and the prohibitive costs associated with high-performance catalysts. Addressing these issues is vital to fully realizing the potential of seawater as a renewable energy resource.
Recent advancements in catalysis have led researchers to explore self-supported nickel-iron (NiFe) materials. These bifunctional catalysts demonstrate high intrinsic activity coupled with affordability, making them suitable candidates for both hydrogen evolution and oxygen evolution reactions. This innovative material combination supports the efficient electrolysis of seawater, overcoming the limitations posed by traditional catalysts. However, ensuring the stability and longevity of NiFe-based electrodes under harsh seawater conditions remains a pressing concern.
A notable development in the quest for effective catalysts is the utilization of wood-based carbon (WC) structures. These substrates provide a naturally hierarchical porous framework that enhances conductivity and overall performance of the catalysis. Their inherent properties not only support the active materials but also contribute to improved electrochemical responses, creating a favorable environment for the complex reactions involved in seawater electrolysis. The approach of using sustainable materials, such as wood waste, underlines the potential for environmentally friendly practices in catalyst development.
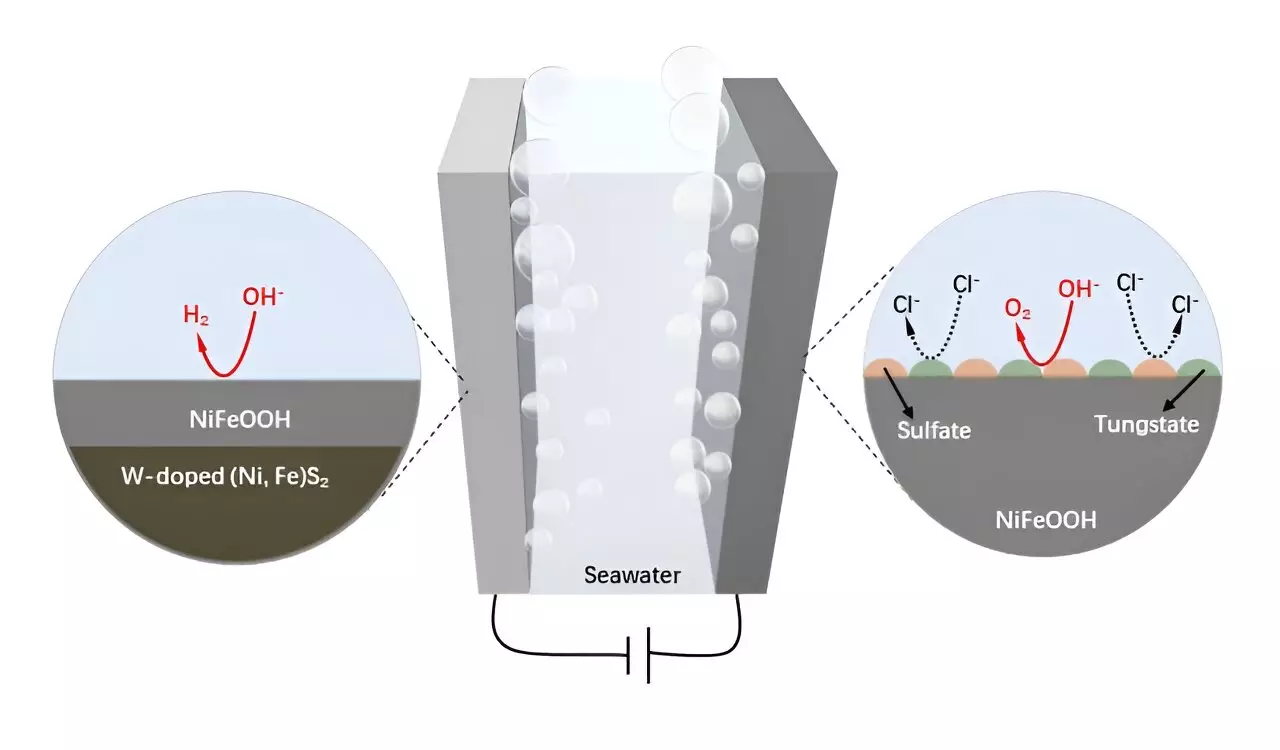
Recent research spearheaded by esteemed scientists from Southern University of Science and Technology, University of New South Wales, and Curtin University has pioneered a novel method by integrating tungsten into NiFe-based catalysts. This modification significantly enhances anti-corrosion properties and improves the stability of the anodes during electrolysis. The introduction of tungsten into WO3-NiFeS/WC electrodes has led to the emergence of a sophisticated three-dimensional hierarchical structure. This design is characterized by oriented microchannels and densely fixed nanoparticles, greatly improving electrical conductivity and catalytic efficiency.
The innovation has culminated in the development of the W-NiFeS/WC electrode, which demonstrates extraordinary performance in both oxygen evolution and hydrogen evolution reactions. Notably, this new electrode excels under alkaline seawater conditions, showcasing robust activity and stability that surpasses traditional catalysts. Researchers have observed that in situ structural evolution during the oxygen evolution reaction generates protective tungstate and sulfate species, further enhancing the electrode’s resilience in a corrosive environment.
The implications of this research extend beyond technical advancements; the use of wood waste-derived carbon delineates a pathway toward a circular economy. By transforming abundant materials into efficient catalysts for seawater electrolysis, this work not only minimizes waste but also champions the sustainable production of green hydrogen fuel. The ability to reuse natural resources exemplifies a forward-thinking approach to energy production, ensuring that sustainability remains a focal point in the development of future electrochemical devices. Through these innovative solutions, seawater electrolysis can move closer to being a viable and eco-friendly alternative in the global transition to clean energy.

Leave a Reply