Polymers play an essential role in a wide range of applications, from everyday products to advanced technologies. However, the traditional development of polymers encounters challenges due to the limitations imposed by the chemical composition of the monomers that constitute them. A groundbreaking study by researchers at Scripps Research, published in *Nature Synthesis*, introduces a novel method using nickel as a catalyst to produce unique monomers. This innovation not only paves the way for polymers with enhanced and varied properties but also emphasizes the potential for more sustainable production practices.
To grasp the significance of this research, it is vital to understand the foundational elements of polymers—monomers. Just as individual train cars come together to form a train, monomers are the building blocks that link up through various chemical bonds to create polymers. The properties of these polymers owe much to their underlying monomer structures, which dictate the characteristics such as flexibility, strength, and reactivity.
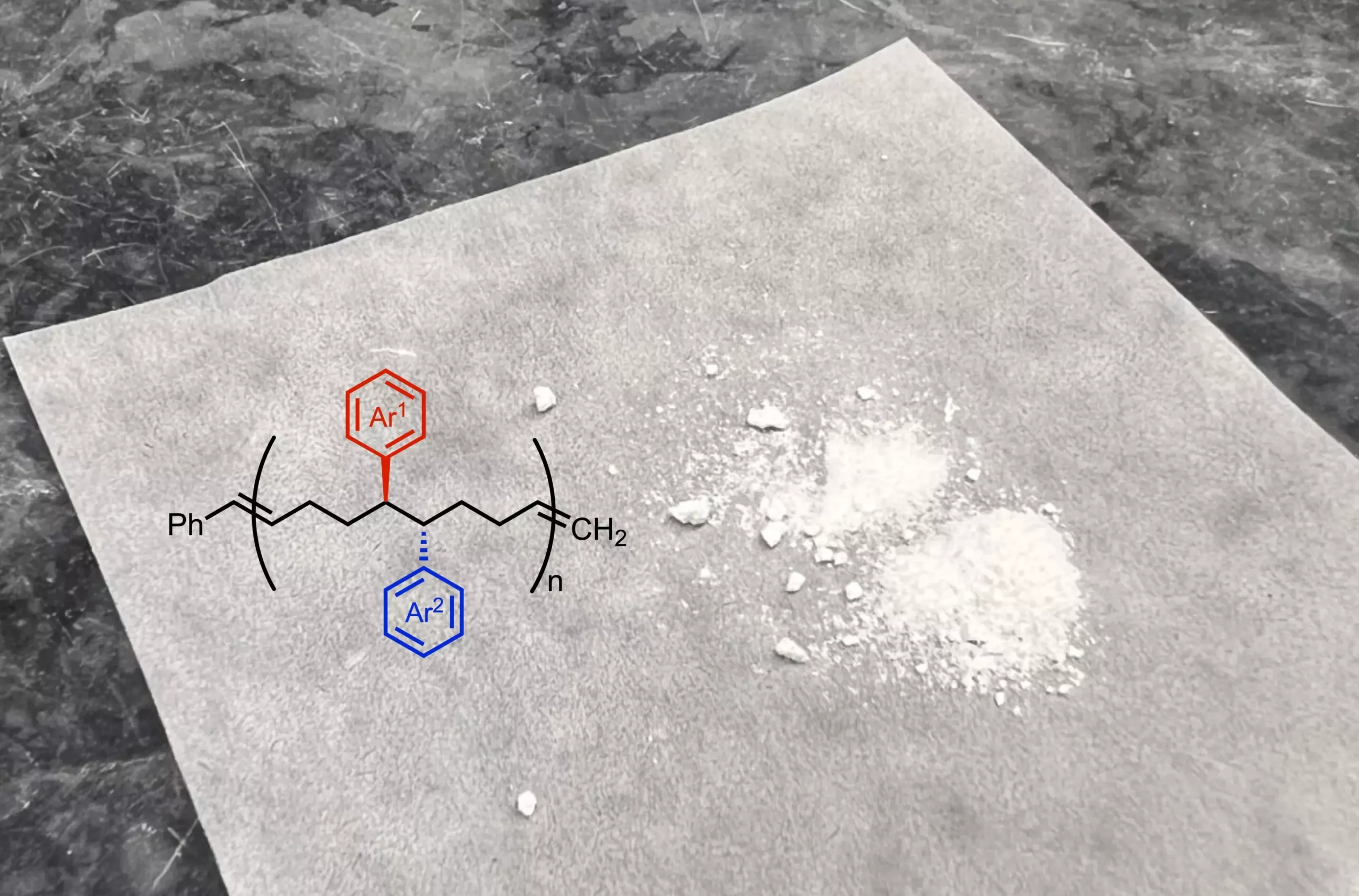
Current polymer productions rely on a limited array of monomers that often restrict the potential functionalities of the resulting materials. Traditional methods produce polymers that may exhibit desirable traits in one area but lack versatility in another. As researchers strive for innovation in fields like drug delivery systems, energy storage, and microelectronics, advancing monomer development is essential for unlocking new operational capabilities in polymers.
In their latest study, Scripps Research chemists, in conjunction with institutions such as the Georgia Institute of Technology and the University of Pittsburgh, have successfully developed a distinct reaction process that enhances monomer structures by utilizing nickel as a catalyst. The innovative reaction incorporates additional functional groups into the monomer framework, thereby diversifying the characteristics that can be imparted to the polymers created from these monomers.
As Anne Ravn, a leading researcher in the study, notes, “If you can modify the chemistry of the building blocks, then you can easily apply it to the macromolecular structure that you’re building.” This capability allows for a significant rethinking of how polymers can be synthesized, enabling researchers to closely control their properties.
A pivotal advantage of the nickel-catalyzed approach is its reliance on an earth-abundant metal. Unlike precious metals typically used in chemical reactions, nickel is not only more accessible but also poses a lower environmental impact. The study emphasizes the importance of sustainability in modern chemical practices, showcasing how utilizing common metals can lead to greener practices in polymer development.
Moreover, this innovative method allows for an increased density of functional groups in the polymer chain, which is a notable enhancement over traditional commercial polymers that often have significant gaps between functional metabolites. Such a configuration promises to yield materials with tailored characteristics, suitable for specific applications ranging from flexible electronics to robust packaging materials.
Looking ahead, the collaborative team plans to investigate the extent to which different functional groups can be integrated into the newly developed monomers. This exploratory phase aims to grasp the relationship between various functional groups and the resultant polymer properties, marking an exciting frontier in material science.
The research team recognizes that the possibility of continual refinement in polymer synthesis grants them unprecedented autonomy in designing materials that can meet the precise demands of various industries. Ravn mentions, “We’re now working to expand the method to explore how introducing other types of functional groups changes the properties of the material.” This continued exploration serves as an invitation for future research that could reshape how polymers function across multiple applications.
Beyond enhancing material properties, this study addresses another pressing issue—environmental sustainability. As polymers accumulate in ecosystems and contribute to pollution, the necessity for sustainable practices becomes urgent. The Scripps Research team is exploring methods to facilitate the degradation of their innovative polymers back to their original monomer forms. This intention to create a recycling mechanism is promising, offering a glimpse into a circular production system where resources can be reclaimed and reused rather than discarded.
The ongoing research at Scripps Research signifies a paradigm shift in polymer chemistry. By leveraging nickel catalysis to develop versatile monomers, the team not only enhances the structural diversity of polymers but also aligns with the growing need for sustainable and environmentally friendly practices in material development. This study may very well lead to the next generation of materials that are as innovative as they are sustainable, challenging the status quo and paving the way for future advancements in polymer science.

Leave a Reply