Solid-state electrolytes have garnered significant attention over the years as a promising alternative to traditional liquid electrolytes used in batteries. These innovative materials are designed to enhance the safety and efficiency of energy storage systems, particularly as the demand for more reliable and longer-lasting batteries increases. The ongoing quest for next-generation battery technologies often leads researchers to explore novel materials that can provide superior performance metrics, including higher conductivity and enhanced stability.
Despite years of research, solid polymer electrolytes have not yet achieved the performance required to make them a viable alternative to their liquid counterparts. Traditional solid polymer electrolytes tend to exhibit low ionic conductivity, which is crucial for the efficient movement of ions within the battery. This issue necessitates the investigation of new approaches in materials science and engineering to improve their characteristics and functional capabilities.
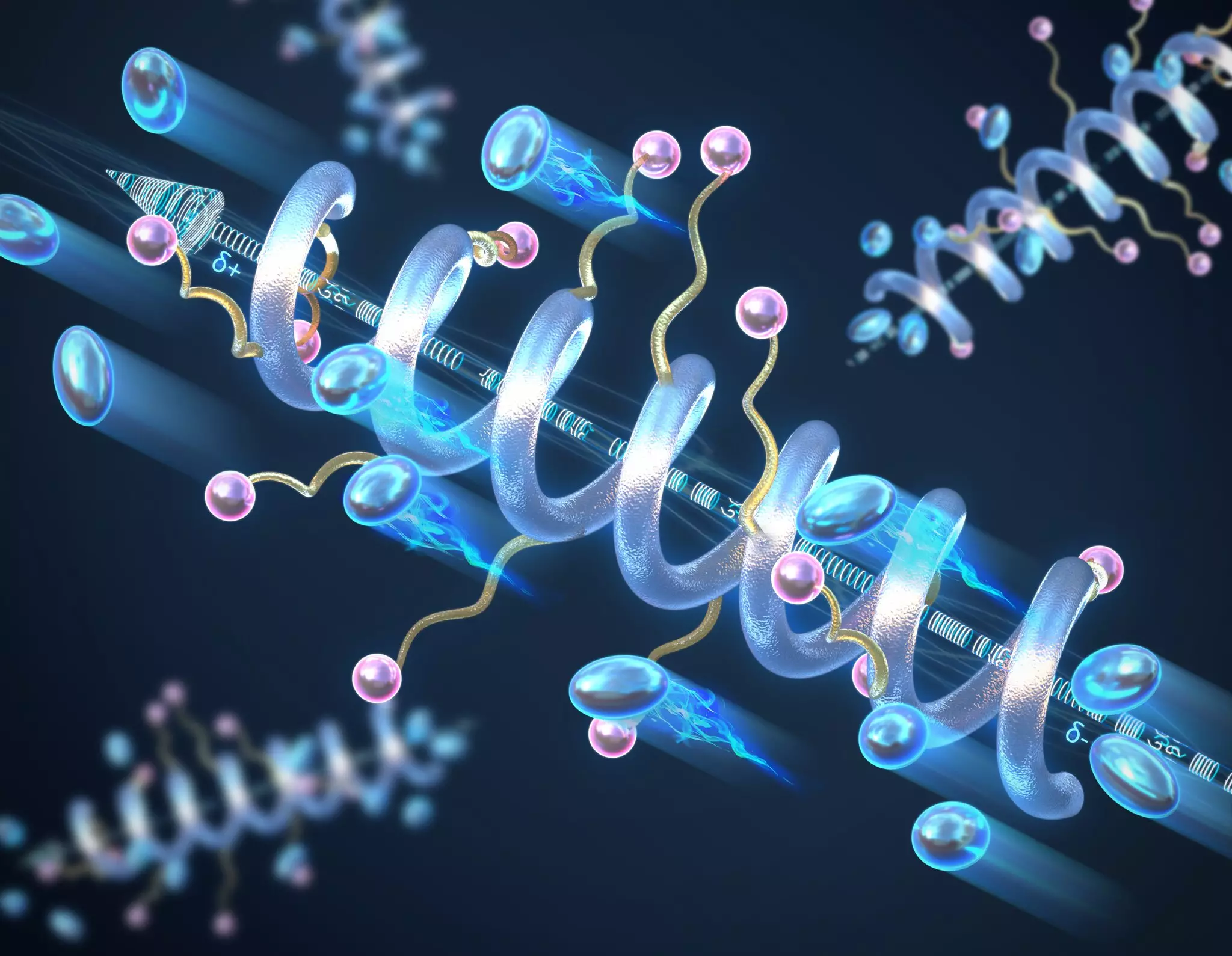
In a recent study conducted by researchers at the University of Illinois Urbana-Champaign, the relationship between helical secondary structures and the conductivity of solid-state peptide polymer electrolytes was examined. The results revealed that introducing a helical structure significantly enhances the ion conductivity compared to the conventional “random coil” structures often found in polymers. The team, led by Professor Chris Evans, posits that longer helices correlate with increased ionic conductivity, which is a critical finding for the development of more effective solid-state batteries.
The Science Behind Helical Structures
Helical structures in polymers are characterized by a specific arrangement that allows for a macrodipole moment to form—an essential feature for ionic conductivity. This arrangement of charged units creates a more favorable environment for charge transport as compared to random configurations typically displayed by polymers. The study reinforces the idea that manipulating the polymer backbone architecture to adopt a helical form can lead to significant advancements in both the conductivity and dielectric constant of these materials, which is vital for their effectiveness in energy storage technologies.
One of the major advantages highlighted by the research is the enhanced thermal and electrochemical stability of helical polymers. These materials not only maintain their structural integrity under higher temperatures and voltage conditions but also exhibit a robustness that is absent in traditional random coil polymers. The longevity and reliability of these new materials could revolutionize battery life cycles, addressing a common issue where traditional polymers often deteriorate under stress, ultimately improving safety and performance.
Environmental Impact and Sustainability
In addition to their superior properties, solid-state peptide polymer electrolytes boast an environmentally friendly aspect. Composed of peptides, these materials can be decomposed into their individual monomer units using biological methods such as enzymatic breakdown or acid treatment after their operational life. This feature enables the recovery and potential reuse of starting materials, positioning them as a more sustainable choice within the context of battery technology.
The study conducted by the Illinois researchers signifies a pivotal moment in the development of solid-state electrolytes. By harnessing the intricate structure of peptides to enhance conductivity and stability, there is potential not only for improved battery performance but also for a decrease in environmental impact. As research in materials science progresses, the knowledge gleaned from these studies will likely catalyze further innovations in creating safer, more efficient energy storage systems, paving the way for the future of solid-state battery technology.

Leave a Reply