The field of synthetic chemistry is at a pivotal juncture, with the urgent need to mitigate the environmental impact associated with traditional chemical processes. One of the primary culprits is the extensive use of organic solvents, which not only contribute to over 80% of the waste generated in chemical reactions but also pose disposal challenges. A breakthrough from researchers at the Indian Institute of Science (IISc) in Bangalore may herald a significant shift in this paradigm. Through innovative micellar catalysis, they have pioneered a method that employs a bio-based surfactant derived from agricultural waste, promising a more sustainable future for industrial chemistry.
In most chemical processes, reactions predominantly occur in liquid phases. While this promotes interaction between substrates and catalysts, the prevalent reliance on non-aqueous organic solvents can lead to unwanted side reactions and the production of hazardous waste. In an increasingly eco-conscious world, the necessity to explore greener alternatives becomes paramount. This not only involves looking into the sustainability of materials used in synthesis but also encompasses the methods employed in conducting chemical reactions.
Historically, synthetic chemists have resorted to using toxic solvents to manage reactions involving sensitive substrates and catalysts. This practice has myriad implications for human health and environmental integrity. Therefore, innovative solutions that minimize or eliminate the use of harmful solvents are critical in pioneering a more sustainable chemical industry.
The research team at IISc has introduced CNSL-1000-M, a surfactant synthesized from cashew nut shell liquid (CNSL), an agricultural byproduct of cashew processing. The raw materials for this surfactant are not only abundant—considering India is the world’s second-largest cashew producer—but also inexpensive to procure. The creation of CNSL-1000-M represents a crucial stride towards achieving a shift away from harmful organic solvents to more environmentally benign methods.
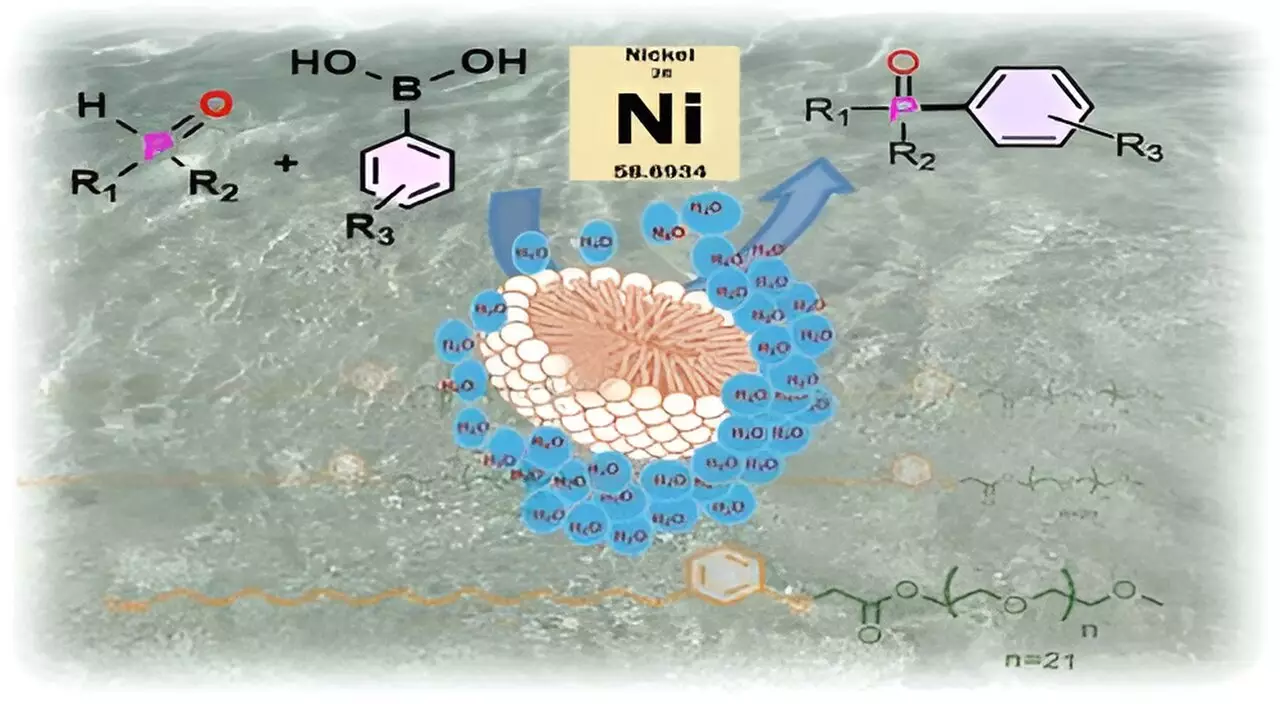
By combining cardanol, a hydrophobic component from CNSL, with m-PEG, a hydrophilic polymer, the researchers crafted surfactant molecules that can form micelles in aqueous environments. This self-assembly leads to the creation of water-free pockets that can encapsulate sensitive substrates, thereby providing a protective environment that mimics the action of biological enzymes, which often sequester their substrates in hydrophobic pockets.
The concept of micellar catalysis is intriguingly analogous to the mechanics of a football afloat in water; as long as the football remains intact, it keeps water at bay. This ingenious analogy helps clarify how the surfactant operates: once the substrate enters the protective micelle, it is shielded from the potentially disruptive effects of water, allowing the desired chemical reactions to occur in an isolated environment.
This methodology has shown remarkable promise, particularly in synthesizing carbon-phosphorus bonds—an essential transformation in the production of several industrially relevant compounds, including pharmaceuticals such as the anticancer drug Brigatinib. Notably, the results from utilizing CNSL-1000-M indicated an impressive increase in product yield, with 80% greater efficiency in water compared to traditional organic solvents. Furthermore, the use of this novel surfactant demonstrated a 30% increase in yields over existing alternatives, all while allowing for the transition from costly palladium catalysts to more affordable nickel-based complexes.
The ambitious goals set forth by the IISc research team reflect a broader trend toward greener chemistry. Their commitment to exploring micellar chemistry further and facilitating its application within various industrial settings underscores the transformative potential of this surfactant. By embracing sustainable methodologies like micellar catalysis, the chemical industry can move towards a model that balances productivity with environmental stewardship.
As industries begin to prioritize sustainable practices, the transition from harmful organic solvents to aqueous micellar technologies could become increasingly viable. The potential to not only enhance reaction efficiencies but also reduce hazardous waste aligns with the growing demand for practices that support sustainable development.
The introduction of CNSL-1000-M represents more than just a scientific advancement; it embodies a crucial evolution toward environmentally responsible practices in chemical synthesis. As the industry begins to embrace these innovative approaches, the future of chemistry looks promisingly greener.

Leave a Reply