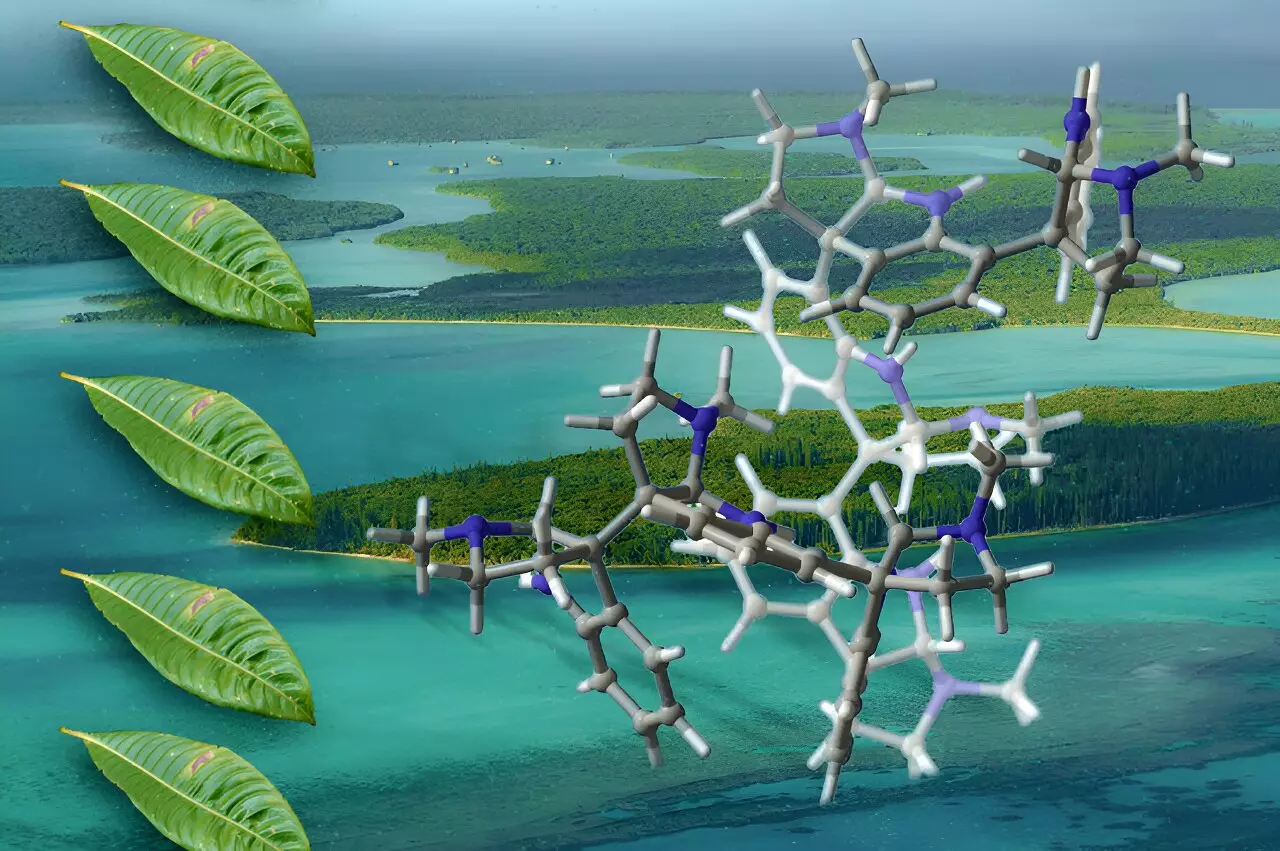
The field of chemistry is witnessing a remarkable breakthrough as a team of chemists from MIT has unveiled a new method for synthesizing complex molecules known as oligocyclotryptamines. These compounds, derived from plant sources, hold promise as potential therapeutic agents, including antibiotics, pain relievers, and cancer drugs. Despite their innovative potential, these molecules have historically proven difficult to synthesize due to their intricate structures and limited natural availability.
Oligocyclotryptamines are fascinating compounds that belong to a class known as alkaloids, characterized by their nitrogen-containing organic structures primarily sourced from plants. Within this family, researchers have identified at least eight distinct oligocyclotryptamines from the Psychotria genus, a group of flowering plants predominantly found in tropical ecosystems. Since the 1950s, scientific inquiry into cyclotryptamines has evolved, revealing the intricate nature of these molecules, which consist of fused cyclotryptamine subunits.
The complexities of synthesizing oligocyclotryptamines arise from their multi-ringed configurations, particularly those with six or seven fused rings. Historically, the synthesis of these larger compounds has remained elusive as scientists grappled with creating stable carbon-carbon bonds in an environment crowded by numerous atoms. The intricacies of their stereochemistry—the spatial arrangement of atoms—further complicate the synthesis process, creating significant barriers that have hindered progress.
The innovative synthesis technique developed by the MIT team overcomes many of these hurdles by streamlining the assembly of cyclotryptamine-derived components. The researchers achieved this by incorporating a technique referred to as diazene-directed assembly, which allows for the controlled formation of carbon-carbon bonds between crowded atoms. This precision is largely attributed to the transformation of carbon atoms into radicals—highly reactive species—enabling them to bond selectively under specific conditions.
The method entails a sequential approach, where a cyclotryptamine derivative is paired with additional cyclotryptamine fragments. The controlled addition of these fragments is fundamental, allowing researchers to manipulate the final molecular structure methodically. The process harnesses the reactivity of carbon radicals formed when nitrogen atoms connected to targeted carbon atoms are liberated. This radical coupling leads to the formation of complex oligocyclotryptamines, granting scientists access to compounds that were previously inaccessible.
The successful synthesis of these oligocyclotryptamines marks a pivotal moment for researchers looking to explore the potential medicinal properties of these compounds. As Professor Mohammad Movassaghi, the senior author of the study, asserts, the newfound ability to reliably produce these molecules could lead to significant advancements in medicinal chemistry. With sufficient material, the research community can carry out comprehensive studies to uncover the pharmacological potential that these compounds may harbor.
Moreover, this methodology presents a pathway for the creation of novel derivatives with enhanced therapeutic properties. Researchers can experiment by substituting different components within the cyclotryptamine framework, potentially leading to new medications that offer improved efficacy or reduced side effects.
The broader scientific community has taken notice of this innovation, with peers recognizing the work as a tour de force in organic synthesis. Leading experts, including Professor Seth Herzon from Yale, emphasize the accomplishment’s significance in the landscape of organic chemistry, highlighting the challenging nature of the task and the ingenuity involved in overcoming longstanding obstacles.
In summation, the MIT chemists’ breakthrough in synthesizing oligocyclotryptamines represents a significant leap forward in the field of organic synthesis and medicinal chemistry. By presenting a novel method for constructing these complex molecules, the team not only opens the door to further exploration of their therapeutic properties but also sets the stage for the development of new variants that may revolutionize current treatment modalities. As research advances, the implications of this work could resonate across pharmaceutical development, potentially leading to new strategies in combatting various health challenges. The meticulous and innovative approach undertaken by Movassaghi and his team exemplifies the transformative power of science in unraveling nature’s complexities and harnessing them for human benefit.

Leave a Reply