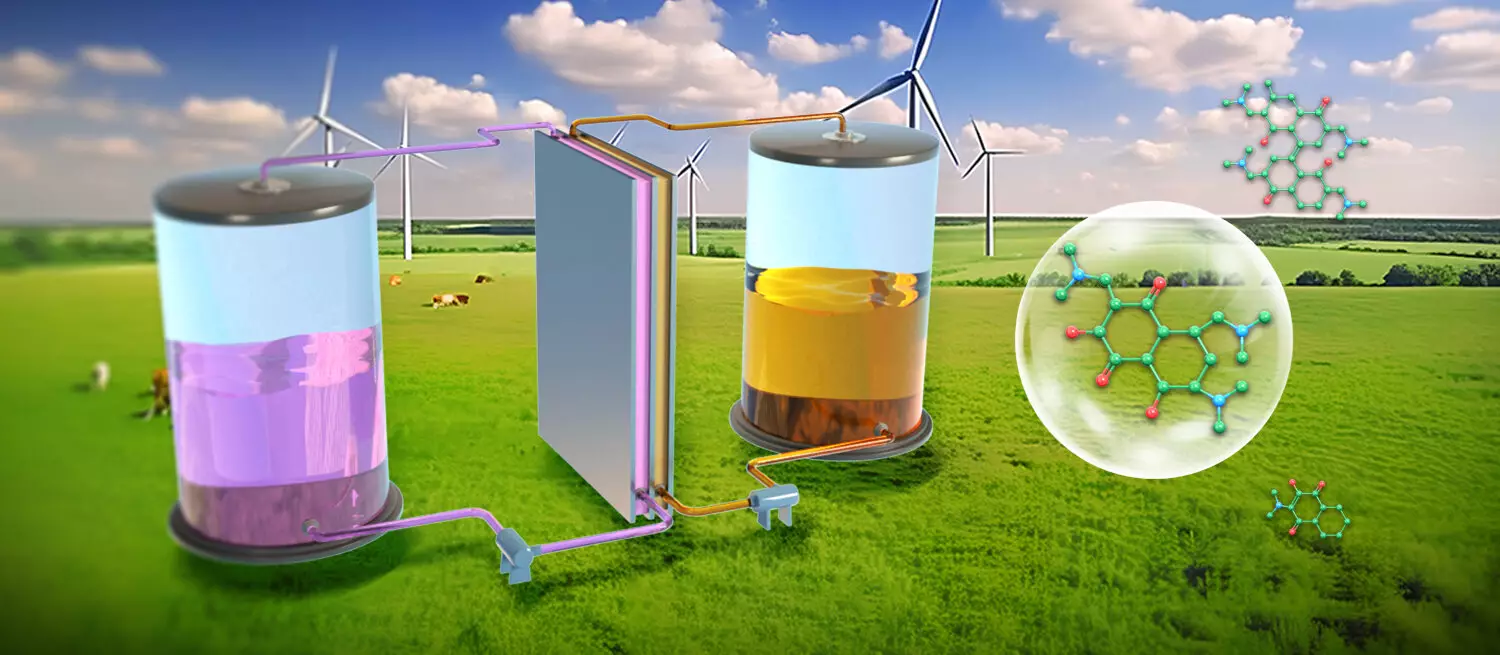
Organic redox-active molecules (ORAMs) represent a vibrant avenue in the field of energy storage technologies, specifically for aqueous organic flow batteries (AOFBs). Their innate diversity and cost-effective nature make them excellent candidates for sustainable energy solutions. However, a significant bottleneck affecting their widespread application is their stability during charge and discharge cycles. Frequent side reactions during these processes can lead to loss of redox activity, making stability a crucial requirement for these molecules.
A recent study conducted by a team of researchers at the Dalian Institute of Chemical Physics, led by Professors Li Xianfeng and Zhang Changkun, addresses this stability issue head-on. They have successfully synthesized novel naphthalene derivatives characterized by active hydroxyl groups and dimethylamine scaffolds, which have shown promise in maintaining stability even in air. This achievement is significant, as air exposure is known to compromise the performance of many ORAMs, hindering their practical utilization. Their findings, published in Nature Sustainability, reveal that these new derivatives can undergo prolonged cycling without succumbing to air-related decay.
The synthesis of these naphthalene derivatives employs a scalable method combining traditional chemical techniques and in situ electrochemical processes. This methodological development not only simplifies the purification of the compounds but also places them on a lower-cost trajectory that is crucial for real-world applications. The structural integrity of the naphthalene derivatives during electrochemical reactions also provides insights into how modifications at the molecular level can enhance performance. The multifunctional framework of these molecules, with a focus on hydrophilic alkylamine groups, helps mitigate unwanted side reactions while improving solubility in water-based electrolytes.
The experimental results are compelling. The 1.5 mol/L naphthalene-based AOFB showcased consistent cycling performance for an impressive 850 cycles over about 40 days, maintaining a capacity of 50 Ah L-1. Even more noteworthy is its capability to operate efficiently under continuous air flow, sustaining approximately 600 cycles over 22 days without any noticeable drop in capacity or efficiency. This characteristic air stability distinguishes the naphthalene-based catholyte from its predecessors, indicating a significant advancement in the viability of ORAMs in real-world applications.
In a notable progression from laboratory to practicality, the researchers were able to scale the production of these naphthalene derivatives to the kilogram level, achieving as much as 5 kilograms per batch. Pilot implementations of battery stacks utilizing these derivatives reported an impressive average capacity of roughly 330 Ah while retaining near-perfect cycling stability over 270 cycles; they recorded a capacity retention rate of 99.95%. This exemplary performance sets a new benchmark in the quest for reliable and efficient energy storage solutions.
The innovative breakthroughs in the development of air-stable ORAMs by the Dalian Institute highlight the potential for transforming sustainable energy storage technologies. The enhanced stability and cost-effective synthesis methods presented pave the way for a new generation of electrochemical energy storage solutions, which could prove instrumental in addressing global energy challenges. The research is not just incremental; it heralds a new frontier in molecular design, promising a sustainable future for energy systems reliant on readily available materials.

Leave a Reply