Lithium-ion batteries are ubiquitous in today’s technology-driven world, powering everything from smartphones to electric vehicles. Despite their extensive use, these batteries come with significant risks, particularly due to the highly flammable nature of their electrolytes. As our dependence on efficient energy storage solutions grows, so does the responsibility to enhance the safety and efficiency of lithium-ion batteries. Recent research from the Martin Luther University Halle-Wittenberg (MLU) unveils a promising innovation: a novel gel electrolyte designed to significantly mitigate risks while improving performance.
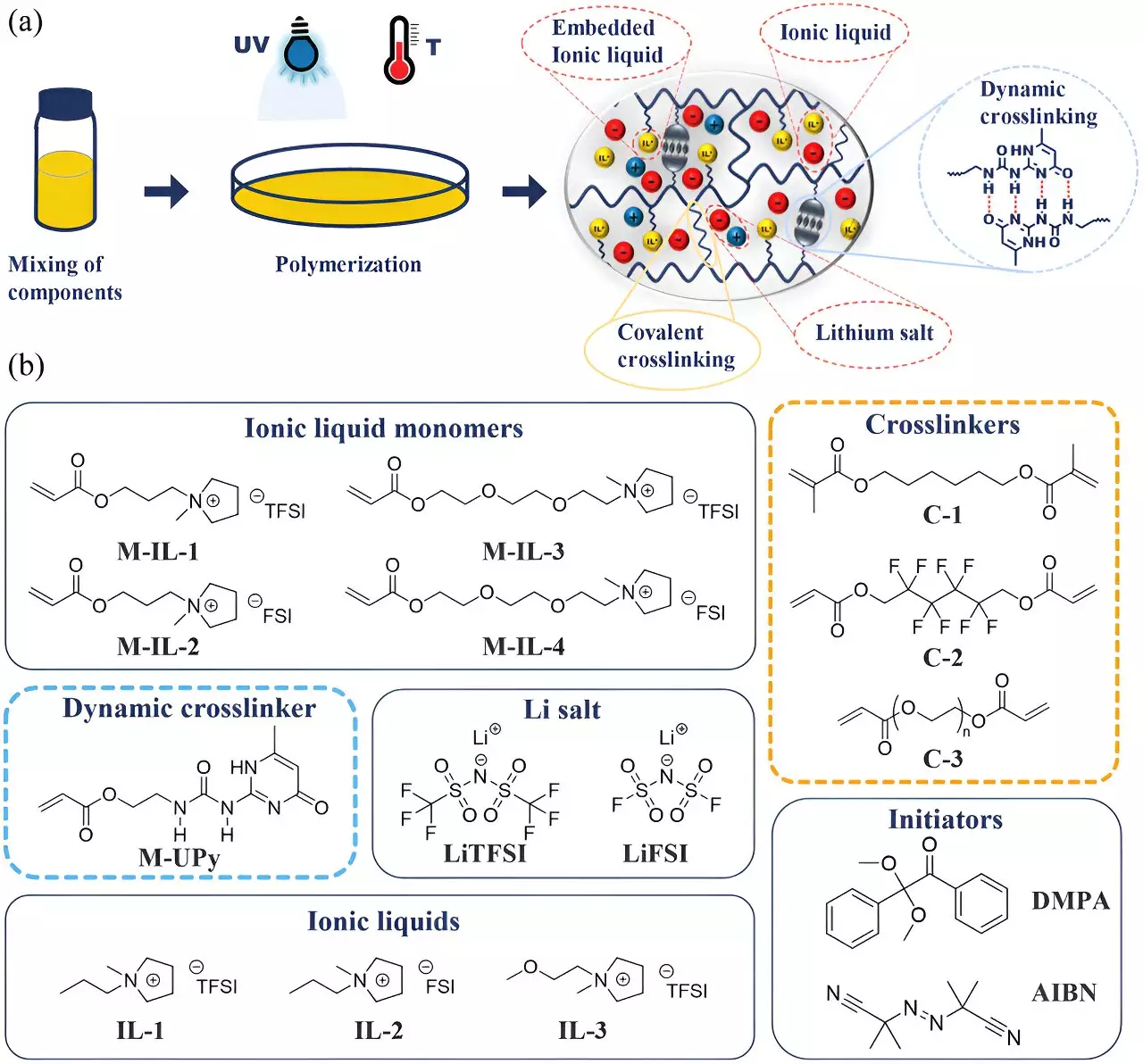
At the heart of this innovation is a polymer-based gel that addresses the core issues of electrolyte leakage and combustion risks associated with liquid electrolytes. Under the guidance of Professor Wolfgang Binder and with contributions from chemist Dr. Anja Marinow, MLU’s research team has pioneered this groundbreaking technology. According to Marinow, the newly developed gel not only encapsulates the electrolyte but also enables ions to flow freely between the electrodes. This dual capability helps maintain the batteries’ performance while enhancing their safety profile against potential hazards associated with traditional electrolytes.
The gel’s consistency is pivotal to its function—delivering the high conductivity typical of liquids while also providing the robustness and thermal stability common in polymers. This combination is not merely an incremental advance; it reimagines the operational framework of lithium-ion batteries, allowing them to achieve safety standards previously deemed unattainable.
Overcoming the Stability Challenges
One of the primary challenges with integrating gel electrolytes into lithium-ion technology stems from the need to establish a stable interface between the electrodes and the electrolyte. In conventional lithium-ion systems, liquid electrolytes form a protective layer during the initial charging process, enhancing battery life and performance. The novel approach adopted by MLU researchers involves embedding an ionic scaffolding within the polymer’s molecular structure, which facilitates this critical stabilizing layer without compromising the gel’s functionality.
Early laboratory experiments reveal that this innovative design not only satisfies safety criteria but also enhances battery endurance and overall efficiency. Real-time data indicate that while typical liquid electrolytes tend to become unstable at around 3.6 volts, the new gel formulation maintains stability at voltages exceeding 5 volts, opening up new avenues for higher-capacity battery designs and applications.
In an era marked by urgent calls for ecological responsibility, this research does not overlook sustainability. The gels developed by the MLU team are crafted to be recyclable, addressing end-of-life considerations for batteries should they be damaged or reach the conclusion of their operational lifespan. This feature exemplifies a forward-thinking approach to battery technology that balances performance with environmental stewardship.
The exploration of these gel electrolytes is part of the wider BAT4EVER initiative, which enlists the collaboration of academia, research institutions, and industry players from across Europe. This cooperative endeavor aims not only to refine the technology but also to integrate sustainable practices into the manufacturing and lifecycle management of next-generation batteries.
Looking Ahead: The Future of Battery Technology
Despite the promising initial results, the journey toward widespread industrial application of these gel-based lithium-ion batteries is far from complete. Long-term testing and investigation will be fundamental to ascertain the comprehensive safety and longevity of these new batteries in varied operational environments. The research propels us closer to revolutionizing energy storage systems while aligning technological progress with the principles of sustainability.
The establishment of the European Center for Just Transition Research and Impact-Driven Transfer (JTC) at MLU is a testament to ongoing commitment on this front. As this center aims to explore pathways for constructive change and sustainable development, it reinforces the crucial role of technological innovation in fostering a circular economy and social advancement.
The strides made by MLU researchers epitomize the next frontier in lithium-ion battery technology, encapsulating our collective aspiration for a safer, more efficient, and environmentally conscious energy future. This development not only promises to enhance the performance of portable energy reserves but also reflects a broader vision of sustainable progress.

Leave a Reply