As the world continues to grapple with the challenges of food security and environmental sustainability, ammonia remains a critical player in both sectors. With an estimated global production of around 175 million metric tons valued at approximately $67 billion, ammonia is essential for fertilizers and various industrial applications. However, traditional methods of ammonia synthesis, particularly the Haber-Bosch process, are energy-intensive and contribute significantly to carbon dioxide (CO2) emissions. This article explores a revolutionary approach to ammonia production through the electrochemical reduction of nitrates, leading to sustainable practices that could reshape this crucial industry.
The Haber-Bosch process, developed over a century ago, relies on high temperatures and pressures to convert nitrogen and hydrogen into ammonia. While it has enabled significant advancements in agricultural productivity, it is not without drawbacks. The process is highly energy-consuming and results in a considerable amount of CO2 emissions, contributing to climate change.
Efforts to mitigate these environmental impacts have led researchers to seek alternative nitrogen sources. One promising candidate is nitrate (NO3-), which is more abundant and easier to reduce than molecular nitrogen (N2). This shift from nitrogen to nitrate could provide a pathway to not only increasing ammonia production efficiency but also addressing environmental issues related to nitrate runoff in water systems.
Recent research conducted by Hao Li and his team at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR) has revealed a promising electrochemical method for synthesizing ammonia from nitrate. Published in the journal Advanced Science in August 2024, the study outlines how catalytic processes can be reimagined to improve both efficiency and sustainability.
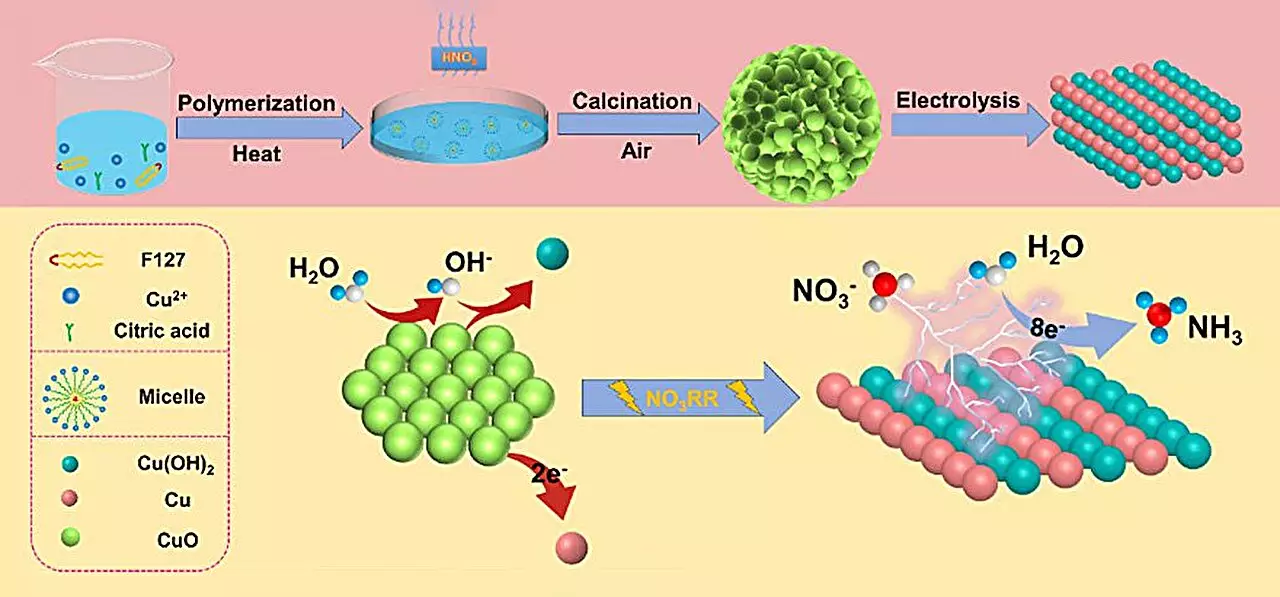
Central to the innovation is the development of a spherical copper (II) oxide (CuO) catalyst. This catalyst displays remarkable performance, with an ammonia yield of 15.53 mg h-1 mgcat-1 and a Faraday efficiency of 90.69% when subjected to a standardized electrochemical setup. The structural characteristics of the CuO catalyst, such as its unique stacking of particles and diverse oxygen states, are essential for its high performance.
Li and his colleagues have identified that the nitrate reduction reaction (NO3RR) facilitates ammonia production through a more accessible mechanism than traditional nitrogen reduction reactions (NRR). The lower dissociation energy and greater solubility of nitrate in water enable a more efficient conversion process. This efficiency is critical in light of increasing global demands for ammonia production and the need for environmentally sound practices.
Moreover, studying the electrochemical behavior of the catalyst revealed structural transformations during the reaction. Specifically, the CuO converts into a composite structure of Cu/Cu(OH)2, which significantly boosts the catalyst’s active sites and promotes better electron transfer. This observation has significant implications for catalyst design, highlighting how structural changes are pivotal in enhancing electrochemical performance.
The research team utilized density functional theory (DFT) calculations to analyze the underlying mechanisms governing these catalytic transformations. The findings suggest that the formation of Cu(OH)2 reduces energy barriers associated with nitrate adsorption, thereby enhancing the overall efficiency of the process. Additionally, this phase positively inhibits the competing hydrogen evolution reaction, which can undermine ammonia synthesis efficiency.
Such theoretical support for experimental observations bolsters the understanding of catalyst design, suggesting that manipulating reaction conditions can yield catalysts with optimized performance characteristics.
As the world transitions towards greener technologies, the pursuit of sustainable ammonia production processes becomes increasingly urgent. Li and his team are set to delve deeper into understanding the phase transitions of their catalysts during nitrate reduction. By refining the design and exploring various factors influencing catalyst performance, they aim to enhance not only stability but also selectivity and activity.
This research contributes significantly to developing more sustainable ammonia synthesis methods. As they pave the way for buildings blocks of a new ammonia economy, the potential for less carbon-intensive agricultural practices and industrial applications seems promising. Ultimately, this innovative approach ushers in a new era of ammonia production, emphasizing efficiency and sustainability in meeting the future’s demands.

Leave a Reply