Recent advancements in chemical synthesis have unveiled the remarkable potential of dinitrogen (N2), a molecule that constitutes approximately 80% of the Earth’s atmosphere. Researchers at RIKEN have made groundbreaking progress that could reshape the landscape of industrial chemistry by enabling more energy-efficient pathways for synthesizing vital compounds, including pharmaceuticals and polymers. This newfound accessibility to dinitrogen presents an opportunity to streamline chemical reactions that have historically required extensive processing and energy expenditure.
Despite its abundance, dinitrogen has remained underutilized due to its inherently strong triple bond, which presents a significant barrier to its direct application in chemical reactions. Traditional methods, such as the Haber–Bosch process, typically convert dinitrogen into ammonia before it can be employed in further reactions. This not only complicates the synthesis by introducing multiple steps but also increases the energy costs associated with producing the necessary nitrogen and carbon sources.
The conventional processes for synthesizing alkyl amines, crucial for various applications, have been fraught with inefficiency. Researchers are aiming to bypass the traditional routes, which involve converting alkenes into more reactive forms, such as alcohols or carboxylic acids, before engaging them in nitrogen fixation. These steps add layers of complexity and energy demand to industrial processes.
The inefficiencies are exacerbated by the type of energy required for these synthetic reactions, which often relies on high temperatures and significant resource consumption. Given the growing emphasis on sustainable and energy-efficient practices in industrial chemistry, the search for innovative methods that streamline these processes is more critical than ever.
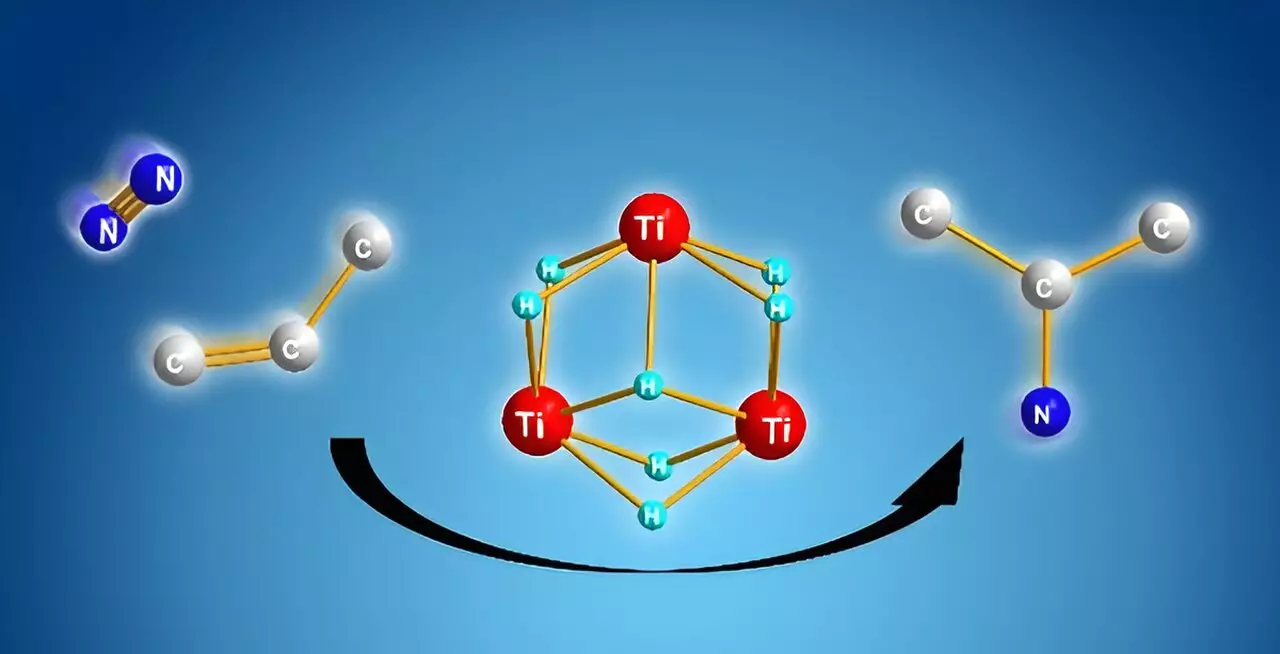
The team at RIKEN, led by Takanori Shima, has explored alternative strategies to circumvent these limitations. Their work with titanium polyhydrides—a novel class of compounds composed of titanium atoms linked by hydrogen—has opened doors to new synthetic pathways. Shima’s team demonstrated that these titanium polyhydrides exhibit exceptional reactivity towards inert small molecules like dinitrogen and benzene, paving the way for efficient alkyl amine synthesis.
Through meticulous experimentation, the researchers established a cooperative mechanism involving multiple titanium–hydride units. This unique interaction facilitates the cleavage of the strong dinitrogen bond while simultaneously activating the carbon source. When alkenes are introduced to the titanium polyhydride framework, they undergo activation, allowing the ambitious reaction to proceed smoothly even under mild conditions.
The crux of this groundbreaking reaction lies in a clear computational analysis. This analysis reveals that the formation of nitrogen–carbon bonds occurs preferentially due to its favorable energy profile when compared to alternative pathways like nitrogen–hydrogen or carbon–hydrogen bond formations. The cooperative activation within the titanium polyhydride framework significantly enhances the overall efficiency and selectivity of the reaction.
As Shima and his colleagues delve deeper into optimizing this synthesis, they are also tasked with turning their findings into a more general catalytic process. If successfully developed, this catalytic transformation could dramatically alter the production of alkyl amines and provide a template for addressing other chemical synthesis challenges.
An Eye on Sustainable Chemistry
The implications of this research reach beyond mere efficiency; they align with broader aspirations toward sustainability in chemistry. With fundamental shifts in how we view and utilize ubiquitous resources like dinitrogen, the potential to reduce our environmental footprint in chemical manufacturing is within reach. As the world grapples with issues of resource depletion and energy costs, innovations like those from RIKEN could lead the way toward a more sustainable future in industrial chemistry.
The pioneering work with titanium polyhydrides and dinitrogen exemplifies how innovative approaches can breathe new life into traditional chemical syntheses. As researchers continue to explore and refine these methods, there is hope for a transformative impact on the chemical industry, underscoring the need for continuous exploration of alternative, efficient pathways in chemical manufacturing.

Leave a Reply