Photocatalysis, a process inspired by nature’s photosynthesis, utilizes light to initiate chemical reactions that would typically require high temperatures or severe conditions. This innovative approach holds vast potential for a wide array of applications, from clean energy solutions to the synthesis of valuable chemicals. However, the widespread adoption of photocatalytic processes hinges on one crucial factor: quantum efficiency. Simply put, for photocatalysis to be economically feasible, the conversion of light energy into chemical energy must be highly efficient.
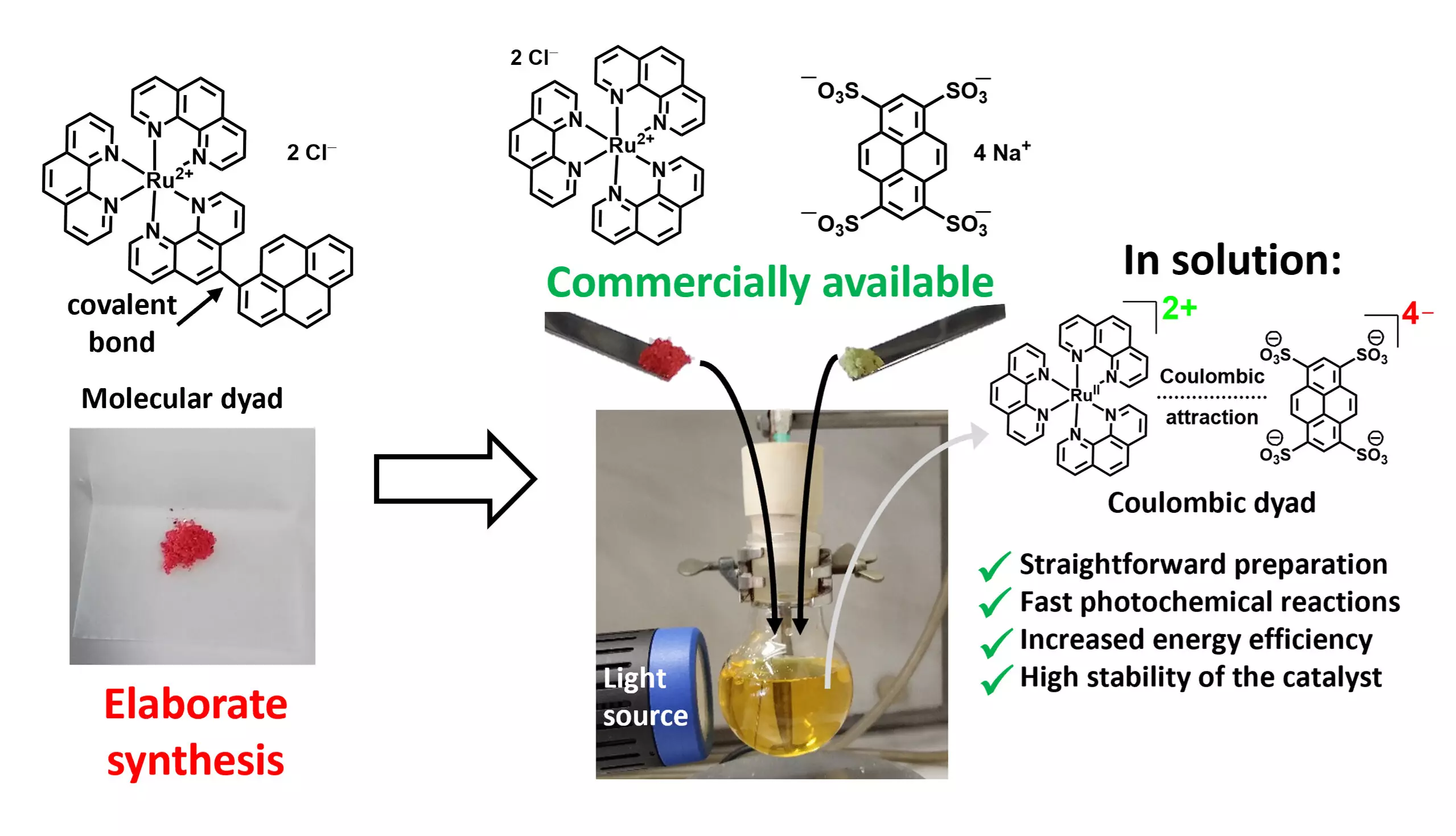
Historically, photocatalysts have relied on intricate designs, often consisting of multiple photoactive components interconnected by covalent bonds, known as molecular dyads. Although these dyads demonstrate remarkable efficiency, their synthesis involves complex and resource-intensive processes, rendering them prohibitively expensive for widespread industrial use. Therefore, the pursuit of cost-effective and efficient photocatalytic materials is a core objective for researchers in the field.
A group of researchers led by Professor Christoph Kerzig from Johannes Gutenberg University Mainz has made significant strides in photocatalysis with a novel approach that simplifies the preparation of efficient dyadic photocatalysts. This groundbreaking method involves mixing two commercially available salts, which, due to their electrostatic interactions—akin to the bonds in table salt—form what are known as Coulombic dyads. Such interactions allow the photoactive components to synergistically enhance their effectiveness in driving chemical reactions, thus improving the overall photocatalytic process.
Matthias Schmitz, the study’s lead author, emphasized the simplicity and effectiveness of their approach, noting the ability to harness established photocatalysts while enhancing performance through inexpensive additives. This advancement shifts the paradigm from developing new catalysts to optimizing existing materials, significantly reducing both resource and time expenditures associated with catalyst preparation. The promise of this method lies in its potential to drastically decrease the amount of catalyst needed, translating to lower costs and improved sustainability.
The identification and optimization of these Coulombic dyads involved an innovative spectroscopy-guided approach, allowing researchers to assess critical steps in the photocatalytic process—from light absorption to the activation of substrates. By utilizing sophisticated laser systems, the Kerzig team could unravel the intricate dynamics at play, thus paving the way for improved catalytic efficiency. Initial experiments demonstrated the viability of these new dyadic photocatalysts in reactions such as carbon-carbon bond formation and the photooxygenation of wood-derived substrates, revealing not only enhanced effectiveness but also a substantial improvement over traditional, more expensive catalysts.
Interestingly, the influence of solvents in photocatalysis emerged as a key factor affecting the performance of these dyads. Researchers have begun to compose a “toolbox” of different photoactive cations and anions, allowing them to tailor the dyadic photocatalysts according to specific solvent conditions. This adaptability could lead to novel strategies for designing photocatalysts aimed at various industrial applications.
The implications of this research extend beyond lab-scale experiments; the potential for industrial-scale photocatalytic applications is vast. By leveraging sunlight and LEDs as energy sources, these efficient catalysts could facilitate the production of high-value chemical products in a sustainable manner. As researchers continue to explore the nuances of these Coulombic dyads, the promise of creating efficient, economically viable photocatalytic processes becomes increasingly tangible.
The work by Kerzig and his team represents a significant leap forward in the field of photocatalysis. By simplifying catalyst preparation through the utilization of Coulombic interactions, they have laid the groundwork for a new generation of photocatalysts that combine efficiency with cost-effectiveness. As this research unfolds, it has the potential to reshape the landscape of photocatalytic processes, fostering a new wave of sustainable chemical transformations that align with contemporary demands for environmental and economic sustainability. As we look ahead, the prospects for photochemistry hold great promise, with this innovative approach at the forefront of potential advancements.

Leave a Reply