As the world transitions toward renewable energy sources, hydrogen has emerged as a potential game-changer due to its clean-burning capabilities. However, despite its environmental benefits, the widespread adoption of hydrogen fuel has been hindered by significant storage challenges. Traditional methods of storing hydrogen, such as compressed gas or liquid hydrogen, demand extensive space and can be cumbersome. Consequently, research communities across the globe are racing against time to devise more efficient solutions for hydrogen storage, and a recent breakthrough from a collaborative team of chemists from prestigious institutions could pave the way.
In a pioneering study, researchers from the University of Hong Kong, Northwestern University, and Duke University have engineered a novel supramolecular material capable of efficiently storing hydrogen in a compact format. This research, published in the esteemed journal Nature Chemistry, employs porous organic crystals that allow hydrogen to be compressed without the excessive weight often associated with storage materials. This leap forward aligns with the U.S. Department of Energy’s critical targets, aiming for a material that holds at least 50 grams of hydrogen per liter while ensuring that the weight of the stored hydrogen constitutes at least 6.5% of the total weight.
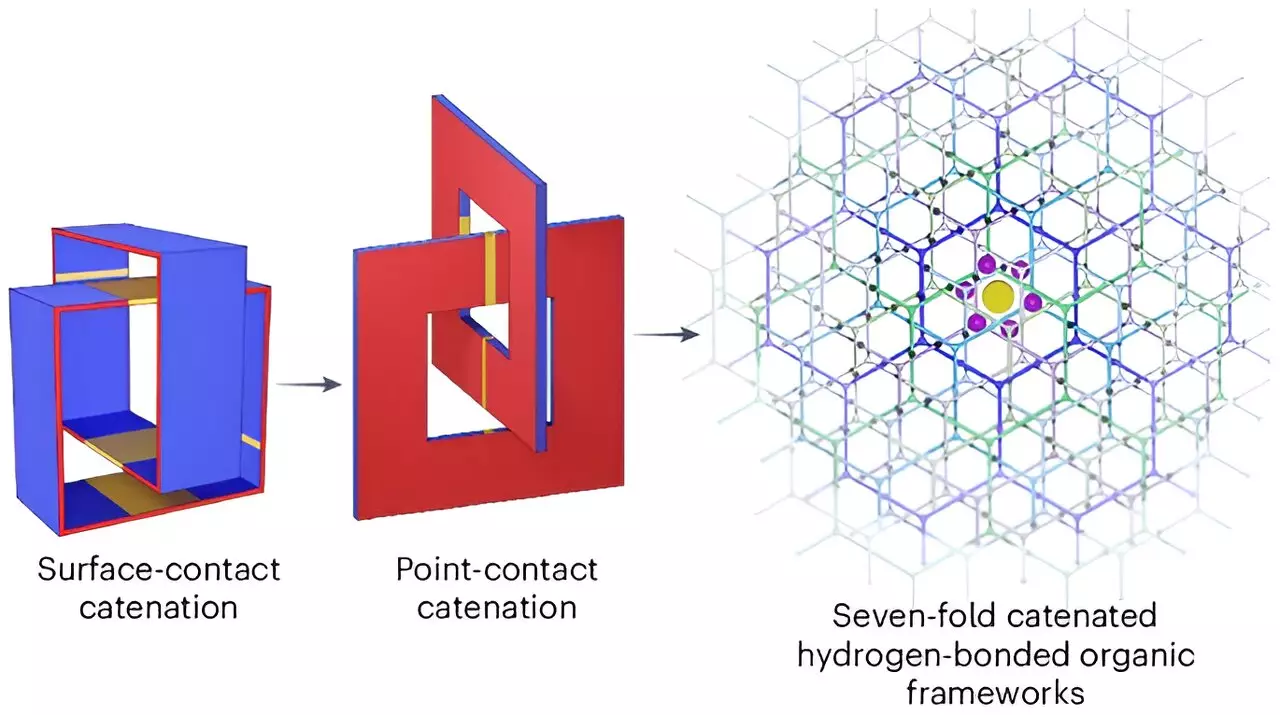
The methodology behind this innovation involves the use of organic molecules arranged in a robust honeycomb structure, creating perfectly sized pores for hydrogen molecules to occupy. By configuring the material in this distinctive way, the researchers have achieved a remarkable storage capacity of 53.7 grams of hydrogen per liter, with hydrogen making up a commendable 9.3% of the overall weight of the storage system. This marks a significant improvement over previous attempts that have struggled to concurrently meet efficiency and weight specifications.
The fundamental advantage of this new supramolecular material lies in its ability to establish strong bonds with hydrogen molecules, promoting a level of stability and density in storage that is unprecedented in prior methods. The interlinked crystal configuration reduces the porousness that has traditionally plagued other materials, thereby enhancing performance and integrity. However, while the findings are promising, the research team faced a notable limitation: the requirement for cryogenic cooling. This cooling mechanism, while essential for maintaining optimal storage conditions, introduces potential costs and operational complexities that could hinder commercial scalability.
As the quest for efficient hydrogen storage solutions continues, this breakthrough represents a beacon of hope for researchers and industry stakeholders alike. The development of a material that not only meets but exceeds current benchmarks for hydrogen density and weight is a significant stride forward. However, moving from the laboratory to real-world applications will require overcoming logistical challenges associated with cooling and cost. With ongoing research and development, this innovative material may eventually contribute to a hydrogen economy where clean energy becomes a viable alternative to traditional fossil fuels, ushering in a greener future for all.

Leave a Reply