The field of biotechnology is rapidly evolving, driven by the necessity for innovative therapies, particularly lifesaving drug treatments. As researchers aim to understand the intricate workings of biomolecules within living cells, traditional methodologies often fall short in providing quick and reliable results. A recent advancement made by scientists at the National Institute of Standards and Technology (NIST) has opened new avenues in this sphere by employing infrared (IR) microscopy to visualize biomolecules in cellular environments where water previously obscured analysis. This article will explore the significance of this development and its potential implications for the biomanufacturing and pharmaceutical sectors.
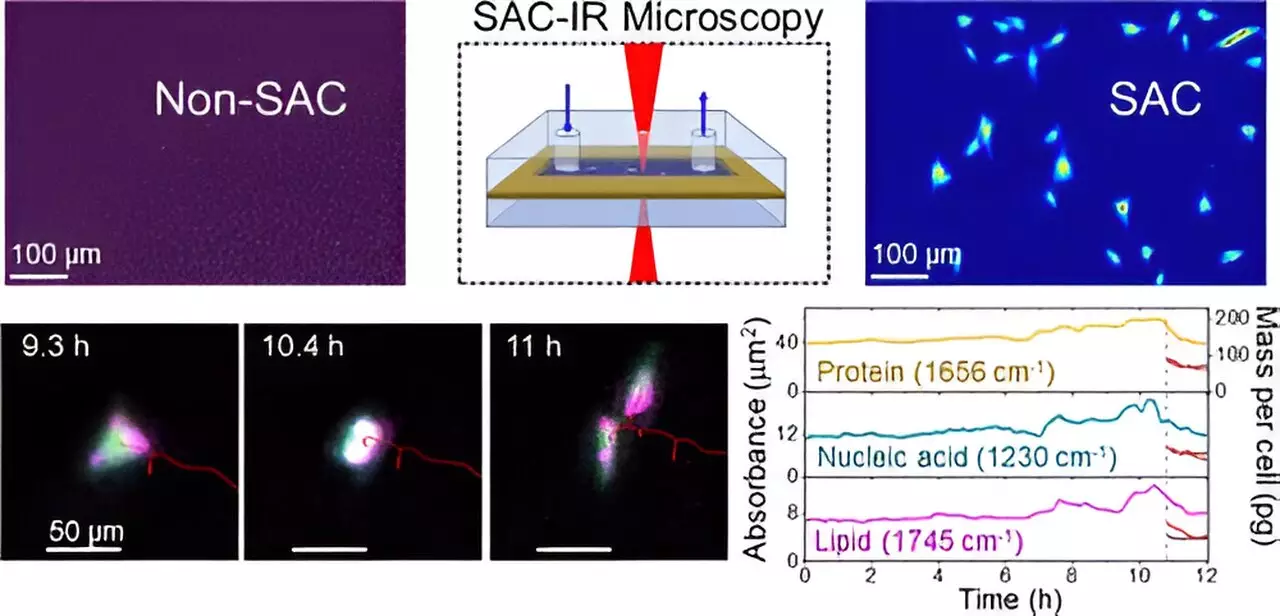
Historically, the presence of water, which constitutes the majority of cellular makeup, has posed significant challenges for IR microscopies. Water tends to absorb IR radiation intensely, creating a masking effect that can obscure signals from proteins and other critical biomolecules. To illustrate this phenomenon, one could liken it to attempting to see an airplane flying under a glaring sun; without the right tools to filter out the sunlight, the object becomes nearly invisible. Recognizing the need for a solution, NIST chemist Young Jong Lee pioneered a method that effectively ‘uncloaks’ the water’s overpowering influence in IR measurements.
The groundbreaking approach developed by Lee, known as solvent absorption compensation (SAC), employs a sophisticated optical system designed to compensate for water absorption in IR imaging environments. This technique was meticulously crafted to work with a custom-built IR laser microscope, demonstrating promising results during a comprehensive 12-hour observation of fibroblast cells, crucial for connective tissue formation. The process enabled researchers to discern specific biomolecular groups—proteins, lipids, and nucleic acids—through various cell cycle stages, all while overcoming the challenges presented by water.
Unlike conventional methods that rely on dyes or fluorescent markers—an approach fraught with variability and potential harm to living cells—the SAC-IR method is inherently label-free. This attribute alone could significantly enhance the reliability of results across different laboratories, establishing a standardized approach for measuring essential biomolecules within cellular contexts.
The implications of this newly developed SAC-IR technique are vast and varied. In the field of cancer therapy, for example, understanding the safety and effectiveness of tissue-modified immune cells is paramount. The precise insights offered by this technique could illuminate biomolecular fluctuations during the modification process, thereby helping assess the efficacy and health of these cells prior to reintroduction into patients.
Furthermore, the technique shows promise in drug screening initiatives. Whether evaluating new pharmaceutical compounds or gauging the safety of existing medications, the ability to measure absolute concentrations of biomolecules across diverse cell populations can dramatically impact drug discovery processes. By providing a more comprehensive understanding of how different cells react to novel drugs, researchers can tailor therapeutic strategies more effectively.
As the NIST research team continues to refine the SAC-IR technique, they aspire to explore its applicability to other vital biomolecules, including DNA and RNA. The expanded capabilities could lead to deeper insights into cell viability, enabling scientists to discern between living, dying, and dead cells with unprecedented accuracy.
An area of considerable interest is the preservation and thawing of cryopreserved cells. As such cells are often stored in frozen conditions for extended periods, the challenge lies in optimizing their revival process to maximize cell viability. The new infrared measurement capabilities could provide meaningful assistance in understanding the impacts of freezing and thawing on cellular integrity.
The revolution spurred by NIST’s advancements in infrared microscopy signifies not only a leap forward in biomolecular analysis but also a pivotal moment for biotechnology and medicine. As methodologies continue to evolve, the integration of novel techniques like SAC-IR may well transform drug development, therapeutic assessment, and our fundamental understanding of cell biology. As researchers pursue further advancements, the potential to unlock new dimensions of cellular analysis promises exciting prospects for the future of health sciences.

Leave a Reply