In the quest for sustainable energy solutions, the oxygen evolution reaction (OER) has emerged as a fundamental process in technologies like water splitting and metal-air batteries. Recent research led by a team of scientists has made noteworthy strides in creating affordable and effective catalysts for this reaction. Their innovative approach incorporates chromium (Cr) into transition metal hydroxides, showcasing promising results that could pave the way for improved energy storage methods.
The process of hydrogen production via water electrolysis has been recognized as a viable means for harnessing and storing renewable energy, particularly from variable sources such as wind and solar. However, the efficiency of these electrochemical systems is often hampered by slow reaction rates associated with the OER. To address this issue, efficient, stable catalysts are essential. The team’s research, published in the journal ACS Catalysis on August 30, 2024, provides insight into how the strategic incorporation of chromium can catalyze these reactions more effectively.
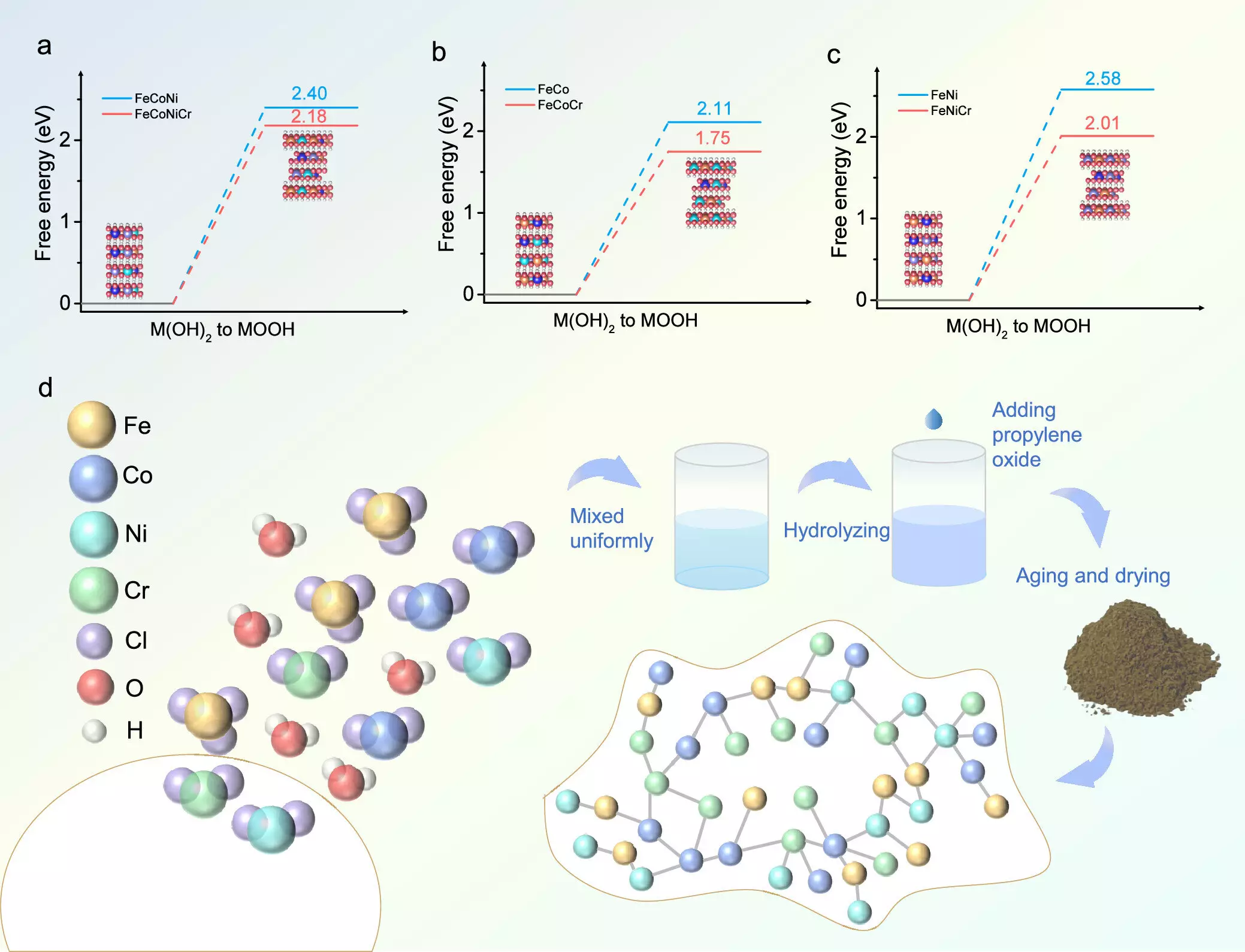
The researchers synthesized a novel FeCoNiCr hydroxide catalyst through an aqueous sol-gel methodology. This technique ensured a uniform distribution of the alloying elements, which is critical for achieving optimal catalytic performance. The developed catalyst exhibited an impressive low overpotential of 224 mV in alkaline solutions—outperforming many existing catalysts by a significant margin. This increase in efficiency is pivotal, especially considering that sustained performance has been validated over an extensive 150 hours of uninterrupted application.
One of the key findings of this research involves understanding how chromium influences the electronic structure of transition metal hydroxides. As pointed out by Hao Li, a co-author from Tohoku University, chromium doping enhances the transition of metal hydroxides to their active oxyhydroxide phase, which is crucial for accelerating OER kinetics. The study also highlighted that theoretical models indicated an optimization of the adsorption energies of important reaction intermediates due to the integration of chromium, thereby facilitating a greater reaction efficiency.
Beyond immediate findings, the research team aims to broaden their investigation to include other elements that could enhance catalyst efficacy further. As discussed by Di Zhang, another co-author, this methodology not only allows for rapid screening of materials but also lays the groundwork for designing superior catalysts. This is particularly exciting in the context of the growing urgency for clean energy solutions as global demand continues to escalate.
The advancements noted in the development of cost-effective and high-performance catalysts for the oxygen evolution reaction reflect a significant step forward in energy research. As researchers continue to explore new materials and methods, there is an overarching goal to bolster the efficiency and durability of catalysts, thereby accelerating the transition to cleaner energy technologies. The findings from this study may represent just the beginning of a broader movement toward sustainable hydrogen production and energy systems, crucial for a greener future.

Leave a Reply