Global warming remains one of the most pressing issues of our time, largely driven by the increasing levels of carbon dioxide (CO2) emissions. In the quest for sustainable solutions, researchers are exploring innovative methods to capture and utilize CO2, particularly in the construction industry. One promising avenue is the process of carbonation in cement-based materials. This process not only addresses the emissions problem but also enhances the utility of materials that hold significant environmental ramifications.
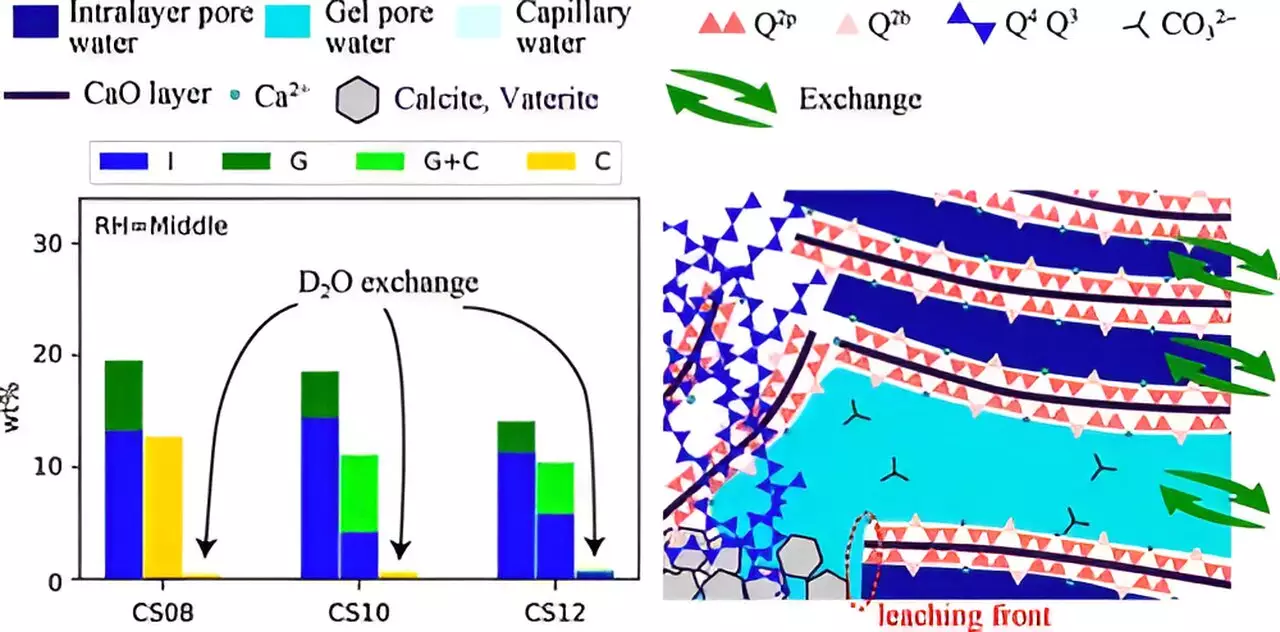
The carbonation phenomenon in cement pastes involves the interaction of CO2 with water, leading to the formation of carbonate ions (CO32-), which subsequently react with calcium ions (Ca2+) derived from the hydration of calcium silicate hydrates (C–S–H), a key component of cement. This process results in the creation of calcium carbonate—a stable mineral form that potentially sequesters CO2. Although exciting, the full mechanistic pathways of carbonation are complex and not entirely understood. Factors like relative humidity (RH), the calcium/silicate (Ca/Si) ratio, and the interactions of ions within nanometer-scale pores influence these mechanisms significantly.
Recent research conducted by a team led by Associate Professor Takahiro Ohkubo at Chiba University has provided deeper insights into these complex mechanisms. Alongside researchers from multiple institutions, Ohkubo’s team investigated the influence of varied Ca/Si ratios and RH on carbonation, releasing their findings in “The Journal of Physical Chemistry C” on July 8, 2024.
Utilizing advanced techniques such as 29Si nuclear magnetic resonance (NMR) and 1H NMR relaxometry, this research marks a significant leap in our understanding of water transport and structural transformations during carbonation. Traditional methods of studying carbonation take decades, as natural carbonation primarily occurs through ambient CO2 absorption. The researchers circumvented this challenge by simulating accelerated conditions using high concentrations of CO2, providing more immediate results about the carbonation process.
The findings of the study reveal critical relationships between the structural integrity of C–S–H and the conditions under which carbonation occurs. It was noted that the collapse of the C–S–H chain structure and the alteration in pore sizes are heavily influenced by both the Ca/Si ratio and relative humidity. Interestingly, lower humidity paired with a higher Ca/Si ratio resulted in smaller pore sizes. This subsequently restrained the escape of Ca2+ ions and water from the interlayer spaces, thereby hampering effective carbonation.
Ohkubo emphasizes that understanding carbonation necessitates looking at both mass transfer and structural adaptations as co-dependent processes. This perspective is critical, as it challenges previous research that might have focused solely on structural changes. The implications extend beyond mere theoretical exploration; they provide foundational knowledge for developing innovative building materials that can significantly enhance CO2 absorption capabilities.
Broader Environmental Context
The potential applications of this research resonate beyond construction materials. The implications extend to environmental science, particularly in understanding how organic compounds in nature also undergo carbonation processes. As society grapples with climate change, the intersection of materials science and environmental chemistry revealed by this research could lead to novel strategies for carbon management.
By integrating insights from the carbonation process into sustainable building practices, it may be possible to produce materials that not only reduce carbon footprints during their production but also continue to sequester CO2 throughout their lifespan. This dual functionality positions carbonation research at the forefront of climate change mitigation efforts.
The study conducted by Ohkubo and his team elucidates the intricacies of carbonation in cement-based materials, offering pathways for significant reductions in atmospheric CO2 levels. This research exemplifies how scientific inquiry can address global challenges by rethinking traditional materials and methodologies. As the pressing need for sustainability in construction becomes increasingly apparent, ongoing investigations into the carbonation process are critical for developing materials that support both the environment and the infrastructure needs of future generations. The findings serve as a pivotal reminder that innovation in materials science can pave the way toward a more sustainable future.

Leave a Reply