Nature possesses an incredible ability to create complex structures from simple components, a phenomenon that is often seen in biological systems. From proteins to viruses, self-assembling entities form critical parts of life. A pertinent example lies within the realm of supramolecular chemistry, which investigates how smaller molecular building blocks can interact to create larger structures with specific properties. This approach not only mirrors biological processes but also paves the way for advancements in materials science.
Supramolecular chemistry concerns the design and construction of large molecules through the deliberate interaction of smaller, predetermined components. By manipulating the affinities between these components—typically polymers—scientists have the ability to create “smart materials,” which can adapt to environmental changes. This dynamic feature of responsive materials is particularly appealing in a world where adaptability can lead to innovative solutions across various fields, from biomedicine to consumer products.
Despite its potential, many fundamental questions remain within supramolecular chemistry. Recent findings published in Scientific Reports by researchers from Osaka University highlight some of these unknowns, particularly concerning the role of additives in microparticle assembly.
Breakthrough Research on Microparticle Assembly
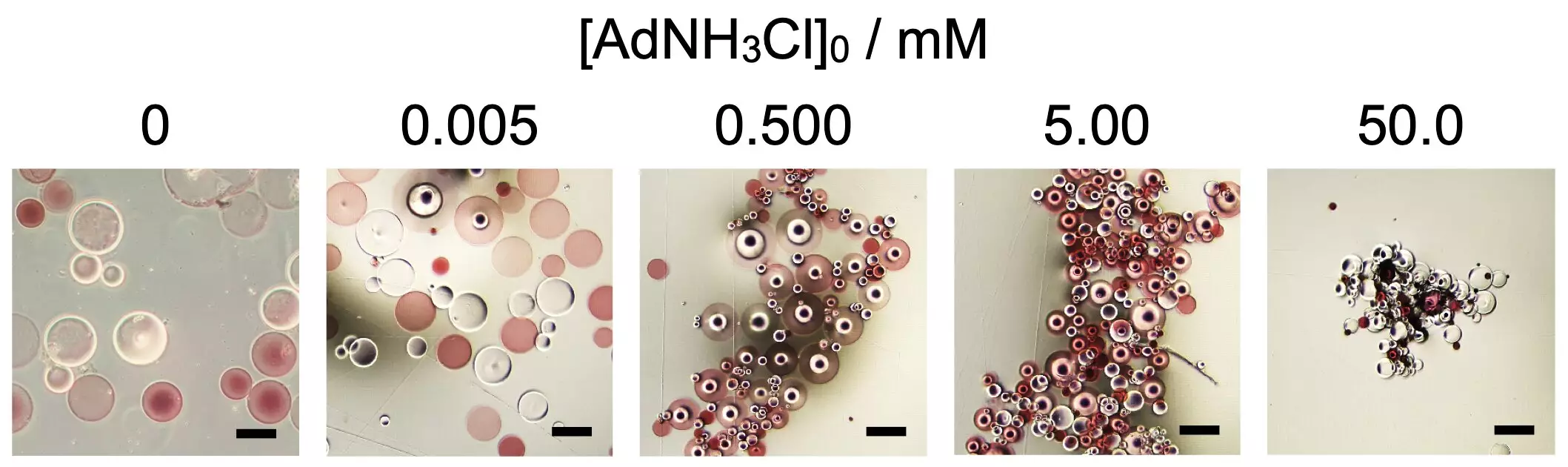
In their study, the Osaka University researchers explored the self-assembly process of spherical microparticles made from poly(sodium acrylate), a super absorbent polymer. The experiment involved introducing chemical additives, specifically 1-adamantanamine hydrochloride, which triggered the assembly of the otherwise static microparticles. Interesting variations in the assembly shapes were observed, dependent on the concentration of the additive, where different configurations ranging from spherical to elongated were achieved.
This nuanced behavior is reminiscent of biological mechanisms, as proteins, composed of amino acids, undergo folding driven by various interactions. Likewise, the interactions among those microparticles suggest that responsive materials could be manipulated similarly, offering exciting possibilities for their practical applications.
The implications of this research extend well beyond academic interest. By revealing how chemical stimuli can dictate material formations, the findings may inform future innovations in active materials that shift their characteristics based on surrounding conditions. This could lead to breakthroughs in sectors like drug delivery systems, where materials could change their properties in response to biological signals.
The notion that the assembly behavior of these microparticles could elucidate the origins of diverse biological shapes adds another intriguing layer to the research. Understanding the mechanisms at play in nature may inspire new designs in technology and engineering, mirroring the efficiency and utility found in biological systems.
The excitement generated by this research reflects a broader movement in science—leveraging nature’s intelligent designs to inspire innovation. By comprehensively studying supramolecular interactions, researchers can open new avenues in material science, leading to smarter, more responsive materials. This exploration not only seeks answers to pressing scientific questions but also drives technology forward, helping to create solutions that resonate with the complexity and functionality inherent in nature.

Leave a Reply