Aluminum oxide, scientifically designated as Al2O3, is a remarkable compound that finds a plethora of applications due to its exceptional insulating properties. It is commonly recognized through its various forms, including alumina, corundum, sapphire, and ruby. This versatility has rendered aluminum oxide pivotal in industries ranging from electronics to catalysis. Recent research has illuminated critical aspects of its surface structure, deepening our understanding of its chemical behavior, especially in catalytic processes. Historically, the precise arrangement of atoms within this material remained an enigma, leading to significant advancements in material sciences.
Traditionally, the atomic arrangement in bulk aluminum oxide is well understood. However, the surface structure has long posed a challenge for researchers. The underlying crystalline lattice of aluminum oxide organizes itself in a predictable manner; yet, the surface, subjected to different environmental influences, behaves differently, leading to a complex and variable atomic arrangement. For over fifty years, scientists struggled to accurately characterize this surface without resorting to invasive techniques, which could alter its properties.
Researchers from TU Wien and the University of Vienna, headed by Jan Balajka and Ulrike Diebold, have recently made a groundbreaking discovery regarding the aluminum oxide surface, resolving a longstanding issue in surface science that had been considered one of its “three mysteries.” This revelation is not only a testament to their perseverance but also a leap forward in our comprehension of aluminum oxide. Their findings have been documented in the prestigious journal, Science.
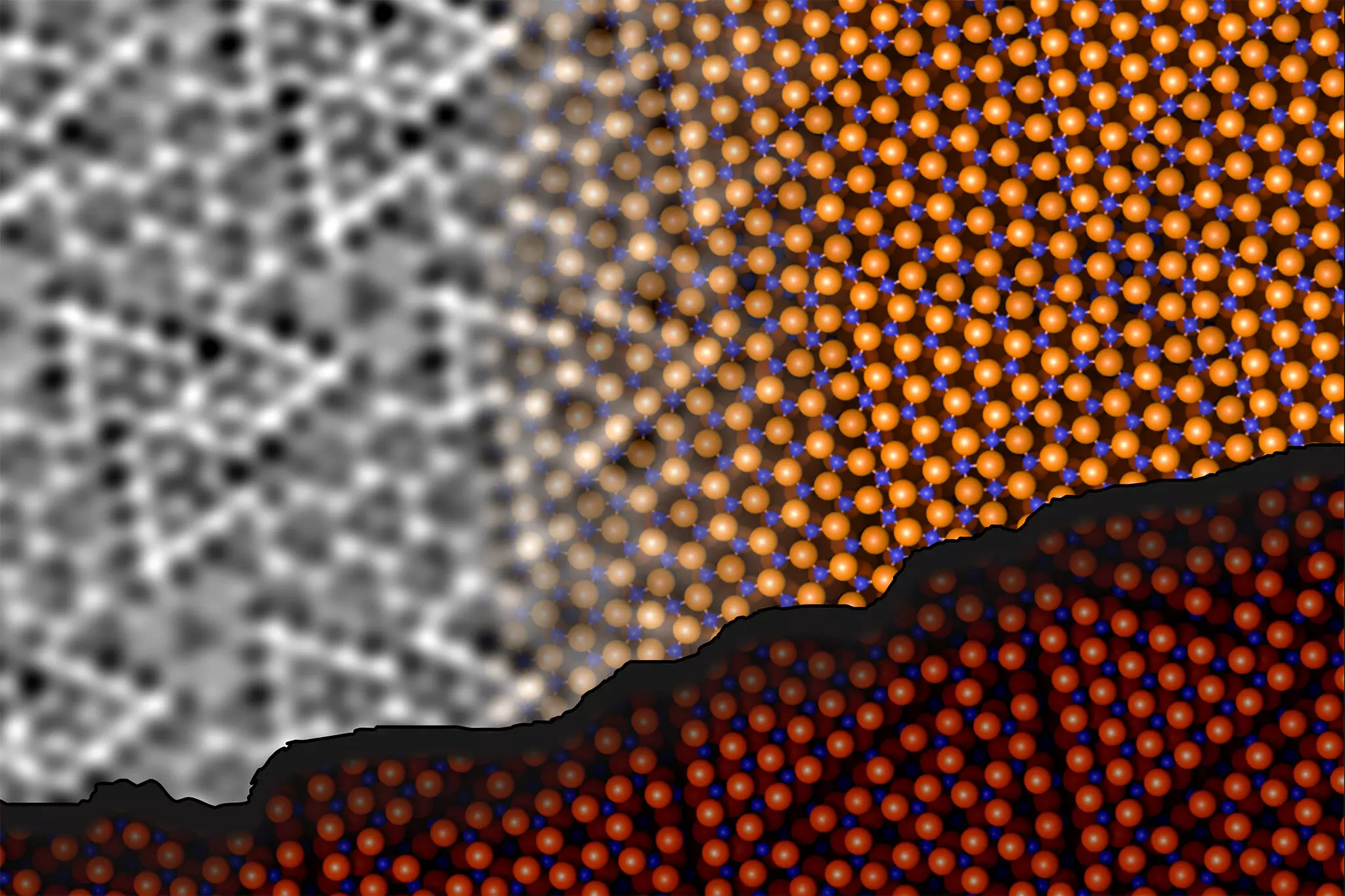
To dissect the aluminum oxide surface structure, the research team utilized noncontact atomic force microscopy (ncAFM), a sophisticated imaging technique that enables scientists to visualize the surface without disrupting its atomic arrangement. This method involves a finely tuned tip that scans the surface at a minute distance. By monitoring the frequency alterations of a quartz tuning fork as the tip interacts with surface atoms, the researchers generated high-resolution images of atomic arrangements.
Johanna Hütner, a key contributor to the experimentation phase, highlighted a significant challenge due to the lack of chemical sensitivity inherent in traditional ncAFM imaging. To overcome this, the team innovatively attached a single oxygen atom to the tip, which allowed them to differentiate between the oxygen and aluminum atoms on the surface based on their unique interactions. By meticulously examining the attractions and repulsions between the tip and surface atoms, the team successfully mapped out the chemical identities of the surface atoms.
One of their most remarkable discoveries involved a reorganization of the surface atoms, wherein aluminum atoms were found to penetrate deeper into the oxide structure, forging bonds with oxygen atoms situated in the underlying layers. This rearrangement leads to a notable decrease in energy, contributing to the stabilization of the overall surface structure. Contrary to prevailing theories, the researchers revealed that the ratio of aluminum to oxygen atoms remained constant, despite this apparent structural transformation.
The construction of a three-dimensional model of the aluminum oxide surface leveraged advanced machine learning techniques alongside conventional computational methods. This integration proved invaluable in tackling the complexity of the surface structure, which presented numerous configurations for the underlying atoms invisible to direct observation. Andrea Conti, a computational modeling expert, emphasized the collaborative success of experimental and computational research in solving this intricate problem.
The research team’s groundbreaking work not only elucidates the intricate nature of aluminum oxide surfaces but also establishes foundational principles applicable to a broader spectrum of materials and their behaviors. The implications of these findings extend into various fields, including catalysis, material science, and electronics, whereby enhanced understanding of atomic interactions could lead to improved material designs and functionalities. Jan Balajka aptly summarized the significance of their investigation, suggesting that their accomplishments may catalyze future innovations across numerous technological domains.
The recent breakthroughs in understanding the surface structure of aluminum oxide underscore the importance of interdisciplinary efforts in advancing material sciences. As research progresses, these insights could facilitate strides in catalysis and pave the way for developing new materials with tailored properties, ultimately enhancing technological advancements in our everyday lives.

Leave a Reply