Recent advancements in biochemistry have unveiled fascinating insights into the functionality of proteins, particularly a protein known as myo-inositol-1-phosphate synthase (MIPS). The research illustrates how MIPS undergoes significant structural changes during its activation process, transforming from a disordered and flexible state to a well-defined structure that carries out crucial functions in metabolic pathways. This study, led by the researchers from Martin Luther University Halle-Wittenberg and the National Hellenic Research Center in Greece, highlights an essential principle in protein science: the structure of a protein underpins its function.
The intricate relationship between a protein’s structure and its abilities has long been an area of focus for biological scientists. It is well understood that proteins are the architects of life, driving growth, metabolism, and various biochemical reactions. When the structure of a protein is even slightly compromised, it can lead to malfunction and, subsequently, diseases in the human body. However, not all proteins adhere to the rigid structural paradigms that have traditionally dominated protein research. Many proteins, MIPS included, exhibit a dynamic character that can complicate their analysis but also offers a deeper understanding of their biological roles.
A Breakthrough Methodology in Protein Analysis
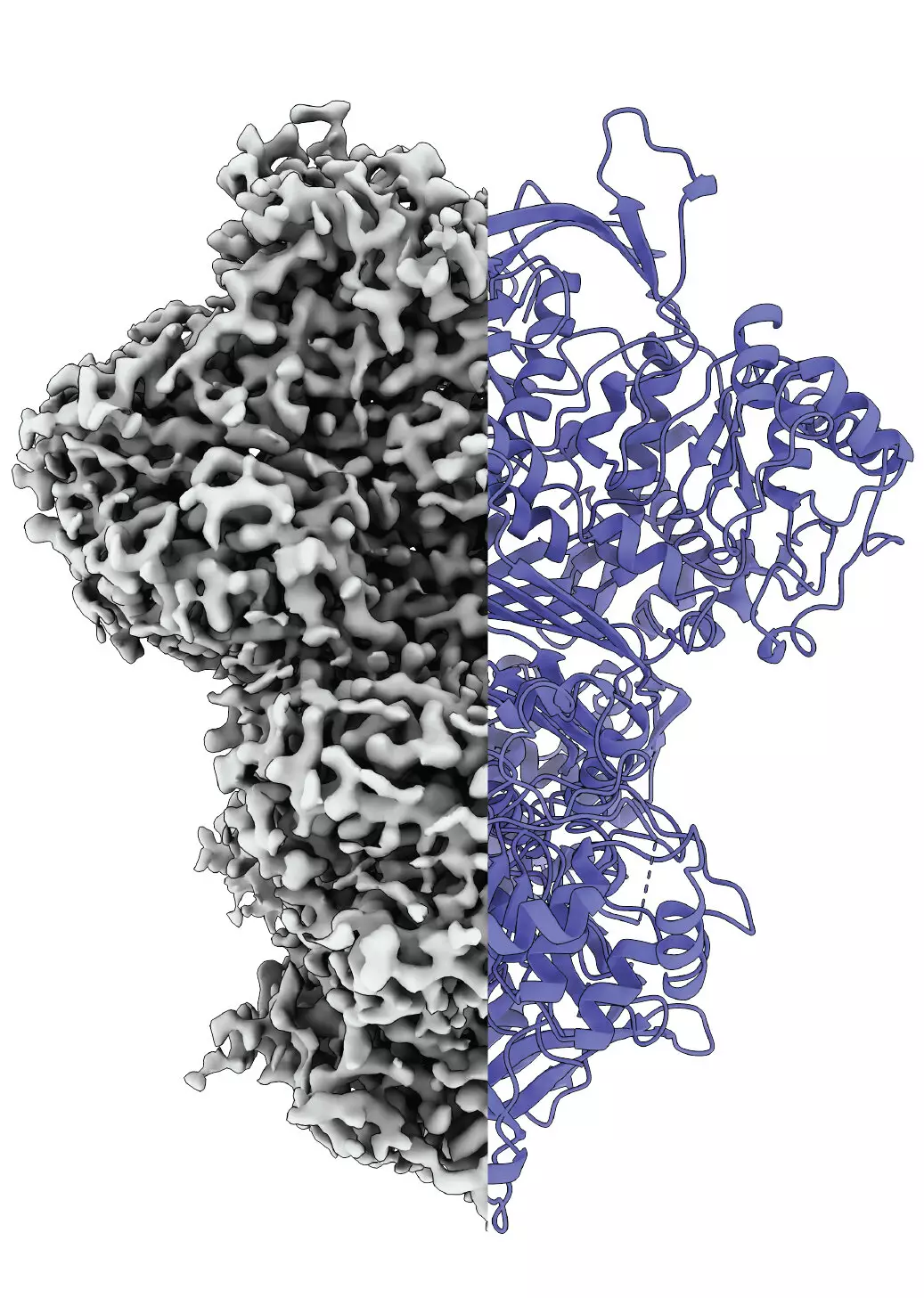
Professor Panagiotis Kastritis and his team have developed a pioneering method that allows for the examination of proteins under near-native conditions, overcoming a long-standing complication in protein research. Conventional methodologies often involve isolating proteins from biological samples, stripping away the environmental context that can influence their structures and functions. By studying MIPS in the context of a model organism, Thermochaetoides thermophila, the researchers achieved clarity in observing how environmental factors impact protein configuration and activity.
Utilizing cryo-electron microscopy, the team delineated three distinct states of MIPS: a disordered state, an ordered state, and an intermediate state. This discovery sheds light on how proteins can transition through various forms depending on their activity and environmental context. The existence of the third state raises intriguing questions regarding its significance—perhaps it aids in the absorption of water molecules necessary for subsequent biochemical reactions or serves an entirely different purpose. These nuanced findings contribute to the growing body of knowledge regarding the complexities inherent in protein dynamics.
Implications for Biomedical Research
Understanding the multifaceted nature of MIPS also opens new avenues for therapeutic development. The insights gained from MIPS’s behavior may not only enhance our grasp of metabolic pathways but also expand the possibilities for addressing metabolic disorders associated with dysfunctional proteins. As Kastritis notes, the foundational knowledge provided by this research could serve as a springboard for future explorations into the mechanisms and functions of related proteins, particularly isomerases, to which MIPS belongs.
The study’s examination of over 340 isomerases illustrates a shared characteristic among similar proteins, one that enables researchers to propose a broader understanding of this family of proteins’ behavior and interactions. This understanding is vital for devising strategies that either enhance or correct protein functions, especially in cases where metabolic pathways are disrupted, contributing to diseases such as diabetes or neurological disorders.
The research on MIPS represents an important milestone in the field of protein science, contributing not only to basic biological understanding but also paving the way for potential therapeutic innovations. By elucidating the structural changes that occur in proteins like MIPS, scientists can glean insights into the underlying principles that govern protein function across a diverse array of biological contexts.
As we continue to unravel the complex lives of proteins, studies such as this exemplify the critical interplay between structure, function, and environmental influence. The quest for understanding proteins in their natural conditions not only stimulates future research but also reinforces the importance of innovative methodologies in advancing the field of biochemistry. The findings may very well herald a new era of therapeutic strategies that are not only reactive but also predictive, improving our approaches to treating diseases rooted in protein dysfunction.

Leave a Reply