Samarium (Sm), categorized as a rare earth metal, has garnered particular attention among organic chemists due to its unique properties, especially the ability of its divalent compounds to perform single-electron transfer reductions efficiently. This capability proves essential in various organic synthesis processes, particularly in the production of pharmaceuticals and other biologically active compounds. However, the common usage of Sm, specifically Samarium iodide (SmI2), presents several challenges, including the requirement for stoichiometric quantities and the use of potentially hazardous chemicals that contribute to the resource-intensive nature of these reactions.
The rising costs associated with Sm and the environmental implications of its chemical applications underscore the pressing need for a more sustainable and efficient catalytic approach. Consequently, a heightened interest has emerged within the scientific community to develop methodologies that minimize the required quantities of Sm, ideally reducing them to catalytic levels while still maintaining reaction efficacy.
Traditionally, Sm-based reactions necessitate considerable amounts—often between 10 to 20 percent—of the rare earth metal relative to the total mass of starting materials. These stoichiometric demands escalate not only costs but also the environmental footprint of these chemical reactions. Despite various approaches aimed at reducing Sm usage to catalytic scales, many of the contemporary techniques either demand aggressive reaction conditions or utilize highly reactive reducing agents. Such requirements contribute to the complexity and potential hazards of conducting these reactions in laboratory or industrial settings.
The landscape of catalytic chemistry is evolving, and there is a significant push to identify alternatives that can alleviate the cumbersome nature of utilizing rare earth elements like Samarium. Achieving sustainable chemistry hinges on devising methods that employ lower amounts of metals while preserving or enhancing reaction efficiency.
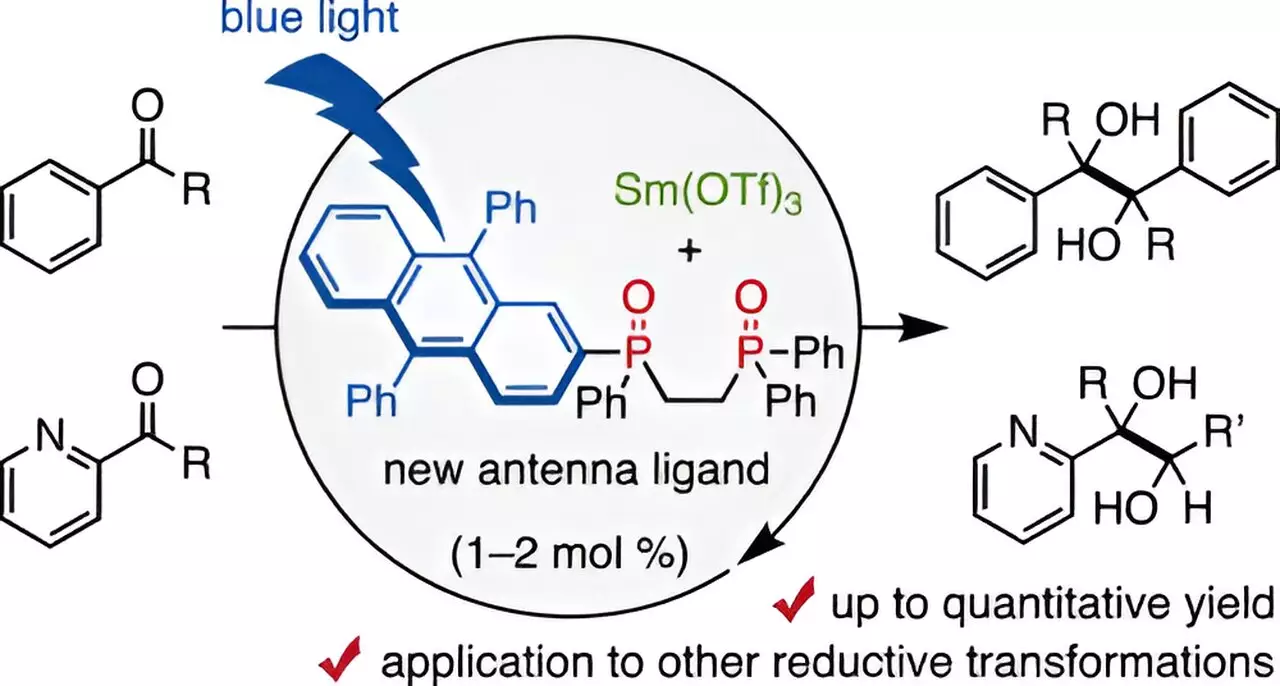
A noteworthy development in the quest for sustainable Sm-based catalysis has emerged from a research initiative at Chiba University in Japan. Led by Assistant Professor Takahito Kuribara, this team has unveiled a novel ligand design that represents a substantial leap in the utilization of Samarium for organic transformations. The innovative ligand, a 9,10-diphenyl anthracene (DPA)-substituted bidentate phosphine oxide, is capable of coordinating with trivalent Samarium and harnessing visible light—identified as a “visible-light antenna” for facilitating reductive transformations.
The ingenious design revolves around exploiting the properties of ligands that can aptly induce electronic excitation of lanthanoid metals such as Sm. The breakthrough, grounded in previous research on alternative DPA-substituted ligands, presents a mechanism whereby minimal amounts of Sm can be engaged in reactions, which could substantially transform the operational protocols in organic chemistry labs.
The experimental results from Kuribara’s research are heralded as promising indicators of potential advancements in pharmaceutical chemistry. Utilizing the DPA-ligand in conjunction with blue-light irradiation resulted in extraordinarily high yields—reaching upwards of 98% in pinacol coupling reactions involving aldehydes and ketones.
This remarkable achievement diminished the requisite Sm catalyst to as low as 1-2 mol%, a dramatic contrast to the previously standard stoichiometric requirements and suggesting a new paradigm in Sm-catalyzed reactions. Additionally, the team discovered that the inclusion of minimal amounts of water surprisingly enhanced reaction yields, although excessive water proved detrimental.
A comprehensive analysis of the interaction between the Sm catalyst and the DPA ligand illuminated crucial insights into the operation of the antenna functionality. DPA-1, the ligand in question, adeptly facilitates charge transfer processes that essentialize its role in the catalytic cycle, thereby setting the groundwork for various molecular transformations critical in drug development.
The innovative approach cultivated by Kuribara and his team represents a significant stride toward more sustainable practices in organic synthesis, particularly in the context of utilizing rare earth metals. As research continues to evolve, this development signals a forward-thinking perspective on integrating green chemistry principles while optimizing the efficiency of Sm-based transformations.
By reducing the dependency on extensive Sm quantities and improving reaction accessibility through visible light activation, the advances achieved in this study provide a valuable blueprint for future catalyst designs in organic chemistry. This research not only enhances the understanding of Sm functionalities but also augurs well for the broader application of rare earth elements in environmentally responsible and economically viable chemical synthesis.

Leave a Reply