A recent groundbreaking study conducted by scientists at Rice University, led by Professor Jason Hafner, has the potential to redefine our understanding of how cholesterol affects cell membranes and their associated receptors. Published in the esteemed Journal of Physical Chemistry, this research not only unveils the complexities of cholesterol within biomembranes but also holds promise for future explorations into diseases connected to membrane organization, including cancer.
Cholesterol serves as a fundamental component in the architecture of biomembranes, which consist of intricate mixtures of proteins and lipids. The role of cholesterol is pivotal; it maintains membrane integrity and fluidity while influencing numerous cellular processes mediated by membrane-resident receptors. Nevertheless, the intricate structural dynamics and interactions of cholesterol within these membranes have historically posed significant challenges for researchers striving to attain a comprehensive understanding.
Innovative Methodologies in Spectroscopy
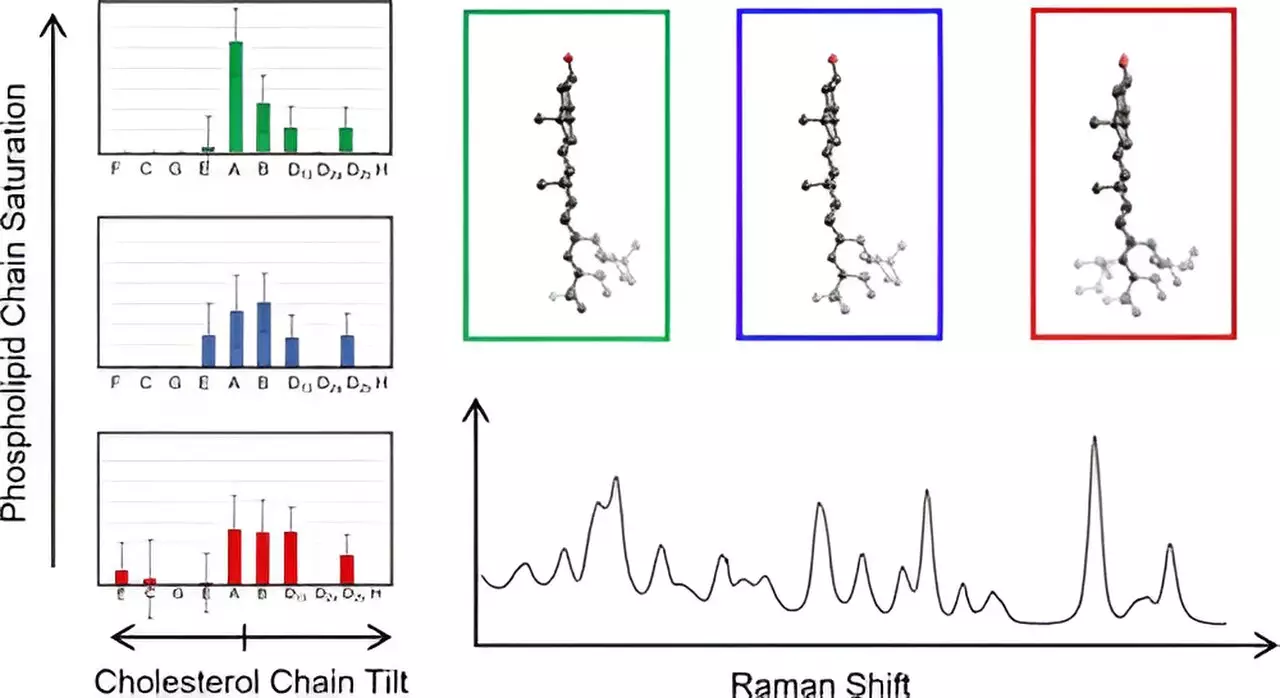
To address this research gap, Hafner and his laboratory employed an innovative approach involving Raman spectroscopy, a technique that leverages laser light scattering to probe molecules at a detailed level. By analyzing the vibrational spectra produced by cholesterol molecules incorporated into membranes, the researchers gathered extensive molecular data. This method, combined with density functional theory—a computational approach rooted in quantum mechanics—afforded the team the opportunity to compare observed and calculated spectra, enhancing their understanding of cholesterol’s structural nuances.
This effort led to the examination of cholesterol’s unique fused ring and its attendant eight-carbon chain. The team meticulously computed Raman spectra for 60 distinct cholesterol structures, uncovering pivotal structural insights that had remained obscured until now. Hafner highlighted the significance of their findings, stating, “This marks the first time we have directly measured cholesterol chain structures in their natural membrane environment.”
New Insights and Future Directions
The implications of this research are profound. During the investigation, the research team found that cholesterol molecules within the same structural category exhibited identical spectra at lower frequencies, streamlining the analysis process and enabling a more efficient mapping of membrane cholesterol chain architectures. Such findings pave the way for a deeper comprehension of cholesterol’s role in membrane organization, which is instrumental in understanding various pathologies, particularly those associated with aberrant membrane function.
One of the standout contributors to the study was Kyra Birkenfeld, a graduate student in physics at Rice, who alongside undergraduate Tia Gandhi and postdoctoral researcher Mathieu Simeral, played a crucial role in the analytical process. Their collective expertise under Hafner’s guidance enhances the study’s credibility and fosters a collaborative environment crucial for advanced scientific inquiry.
The pioneering research undertaken by Hafner and his team offers vital new insights into the structural characterization of cholesterol within cell membranes. This work not only illuminates the role of cholesterol in cellular dynamics but also sets the stage for future investigations into the mechanisms underlying diseases where membrane organization is disrupted. By bridging the gap between molecular spectroscopy and biological relevance, this study points toward exciting pathways for understanding disease mechanisms and developing targeted therapeutic strategies in the realm of cell membrane research.

Leave a Reply