Photosynthesis, a fundamental process through which plants harness sunlight to produce energy, operates on the same principles as photovoltaic cells used in solar panels. Both systems rely on the intricate movement of electrons and the transfer of charge at a molecular level. Understanding the dynamics of these processes is crucial not only in biology but also in the fields of chemistry and materials science, where engineered charge transfer plays a vital role. In this context, a sophisticated understanding of electronic motion driven by light absorption reveals the ultra-rapid phenomena that govern molecular behavior, embodying a blend of quantum mechanics and molecular dynamics.
Researchers have made significant strides in measuring the time it takes for electrons to move and charges to transfer after absorbing photons. The key to studying these events lies in the exceptional precision of temporal measurements. Techniques such as ultrashort ultraviolet pulses generated via high-order harmonic sources or free electron lasers have revolutionized our capability to observe these processes on an unprecedented scale, spanning from femtoseconds (10^-15 seconds) to attoseconds (10^-18 seconds). Each time frame represents a unique window into the world of molecular interaction, offering insights that were once considered unattainable in conventional experimental setups.
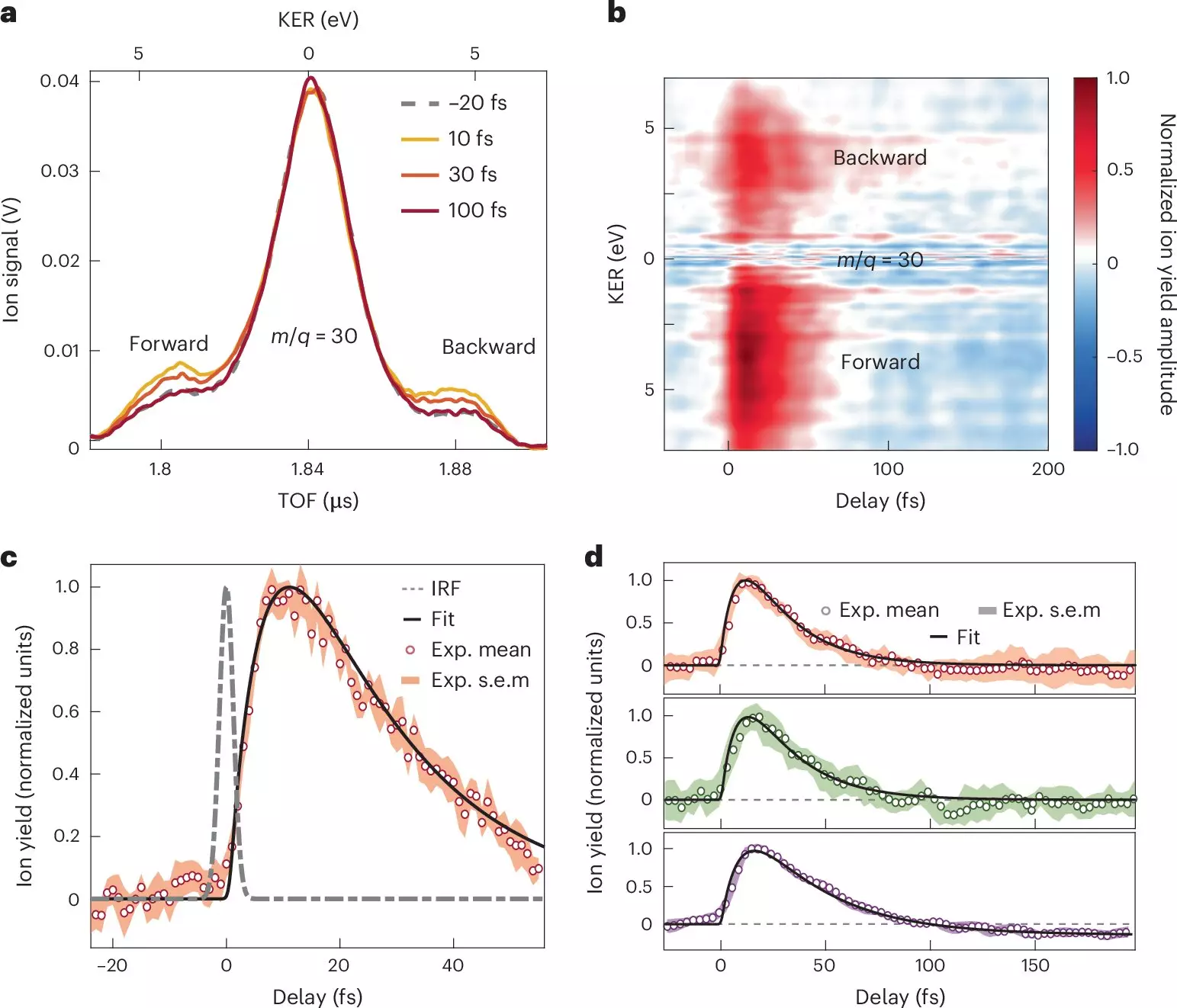
A groundbreaking study published in Nature Chemistry sheds light on these rapid molecular dynamics, with researchers from institutions in Italy and Spain employing attosecond extreme-ultraviolet pulses to probe charge transfer in nitroaniline molecules. By dissecting the earliest stages of charge transfer with extreme precision, this pioneering work enhances our comprehension of the electron-nucleus interactions within donor-acceptor molecular systems.
Through a combination of attosecond extreme-ultraviolet-pump and few-femtoseconds infrared-probe spectroscopy, along with advanced many-body quantum chemistry models, the research unveils the rapid processes that occur immediately after photoionization. The ability to gather precise temporal information about electron transfer dynamics has paramount implications for chemical theory and practical applications.
The team revealed that electron transfer from the amino group donor occurs in less than 10 femtoseconds—a staggering insight that suggests a tightly synchronized interplay between nuclear and electronic motion. Following this rapid transfer, a complex relaxation process begins, unfolding over less than 30 femtoseconds, as nuclear wave packets evolve within the excited electronic states of the molecular cation.
This newfound understanding emphasizes the significance of electron-nuclear coupling, demonstrating its role in influencing the behavior of electron donor-acceptor systems upon photoionization. The study meticulously charts the time required for charge transfer from the donor to the adjacent chemical bond, showcasing the structural transformations involved in these events.
The results presented in this study are not merely incremental; they provide a fresh perspective that enhances traditional diagrams and concepts often utilized to visualize charge migration within organic molecules. This experimental and theoretical foundation paves the way for deeper inquiries into the mechanisms governing charge transfer, promising advancements in both the fundamental understanding of molecular interactions and in practical applications of attosecond science.
As researchers continue to delve into the intricacies of molecular dynamics unveiled by this study, a myriad of applications may become plausible—from the design of more efficient solar energy conversion materials to the development of faster molecular devices. The pioneering work conducted by the collaborative efforts of various research institutions marks a substantial leap forward, opening doors to future investigations in the field of attosecond science and its potential to revolutionize our approach to understanding and harnessing the energy of light.
The investigation of charge transfer dynamics is set to play a central role in the intersection of chemistry, physics, and engineering, potentially leading to breakthroughs that redefine our technological landscape.

Leave a Reply