As the global climate crisis accelerates, the need for innovative solutions to reduce carbon dioxide emissions has never been more pressing. Carbon dioxide, a primary greenhouse gas, predominantly stems from industrial activities and fossil fuel consumption, contributing significantly to climate change. One promising approach to tackle this issue involves capturing CO2 and repurposing it into sustainable fuels. Recent advances from the University of Michigan present a pioneering artificial photosynthesis system, specifically designed to convert CO2 into ethylene, a precursor for plastics and other vital materials. This development not only offers a potential pathway to reduce greenhouse gases but also highlights the potential for recycling carbon emissions into useful substances.
Located at the forefront of renewable energy research, the University of Michigan has engineered a state-of-the-art artificial photosynthesis system that boasts impressive capabilities. Central to this technology is the ability to bind carbon atoms into hydrocarbons with unmatched efficiency, longevity, and yield relative to competing systems. By focusing on the production of ethylene, the team has demonstrated that this innovative system delivers performance that is five to six times superior compared to traditional solar-driven carbon dioxide reduction methods.
Ethylene is recognized as one of the world’s most produced organic compounds, traditionally derived from fossil fuels through energy-intensive processes that generate additional CO2 emissions. This breakthrough approach aims to change the game by utilizing CO2 that would otherwise contribute to climate change, transforming it into a sustainable resource for producing plastics and, eventually, liquid fuels for transportation.
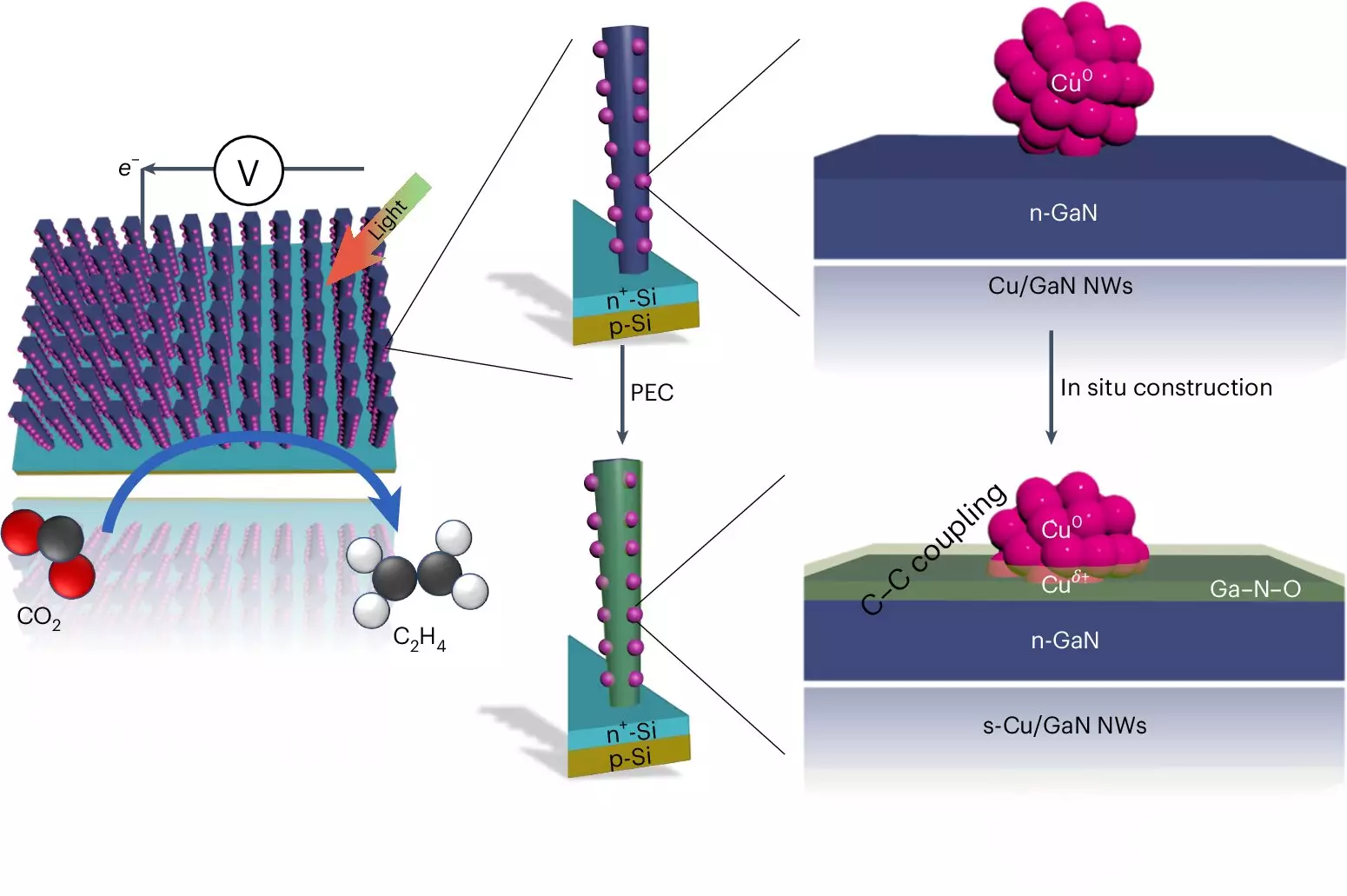
The artificial photosynthesis system employs an intricate process involving two types of semiconductors, each contributing to the overall efficiency. Gallium nitride nanowires, which are minuscule structures just 50 nanometers wide, are combined with a silicon substrate to create a robust platform for chemical reactions. The system operates in a water environment enriched with CO2, where exposure to sunlight catalyzes a series of reactions.
Initially, light energy is absorbed by the nanowires, resulting in the excitation of electrons that energizes the splitting of water molecules. This creates hydrogen, which is essential for the subsequent formation of ethylene, while releasing oxygen that reacts with the nanowires to form gallium nitride oxide. Meanwhile, copper clusters present at the reaction site play a crucial role in capturing hydrogen and converting the CO2 into carbon monoxide. The presence of hydrogen ultimately facilitates the bonding of carbon monoxide molecules, leading to the creation of ethylene.
The innovative aspect of this system is its efficiency: the researchers found that an impressive 61% of the free electrons generated through the light reaction actively participated in producing ethylene. This marks a significant advancement compared to other systems that have historically needed more complex and less efficient processes.
One of the standout features of the University of Michigan’s artificial photosynthesis system is its remarkable longevity. While many alternative catalysts operate effectively only for short periods, often degrading in performance, this system has demonstrated consistent output over extended operational timelines. The device managed to maintain its performance for up to 116 hours without any decrease in efficiency, indicating robust stability—an aspect crucial for practical applications.
In addition, the ongoing research suggests the possibility of extending the lifespan of the device further. Early results indicate that previous iterations of similar systems have operated smoothly for as long as 3,000 hours. This durability stems, in part, from the beneficial interactions between gallium nitride and the water-splitting process, which result in a self-healing capability that reinforces the overall performance of the system.
The ultimate aspiration for the University of Michigan’s research team is far-reaching: to synthesize longer chains of hydrocarbons, including fuels like propanol or other viable liquid fuels. These liquid products could significantly enhance the sustainability of existing transportation technologies by providing a carbon-neutral alternative to conventional fossil fuels.
Bingxing Zhang, assistant research scientist and lead author of the study, emphasizes that the quest does not end with ethylene. Future research is focused on broadening the system’s output to encompass a greater variety of carbon compounds, paving the path for a multi-faceted approach to carbon capture and recycling.
The breakthrough at the University of Michigan represents a critical step toward realizing a future where carbon emissions are not merely an environmental liability but a valuable resource. The implications of this research could transform industries, offering a cleaner, renewable way to produce essential materials while significantly mitigating the impacts of climate change. The vision of sustainable fuels derived from CO2 is not just a dream; it is rapidly becoming a reality.

Leave a Reply