Lithium-metal batteries have long been considered a promising next-generation battery technology due to their potential for high energy density, surpassing that of lithium-ion batteries currently dominating the market. However, these revolutionary cells have been plagued by limitations, most notably a short cycle life. Researchers at University of Science and Technology of China, in collaboration with other institutes, have recently introduced a groundbreaking electrolyte design that could pave the way for highly performing lithium-metal pouch cells with extended lifespans.
A New Approach to Addressing Degradation Issues
One of the primary challenges faced by lithium-metal batteries is the growth of lithium dendrites, along with the high reactivity of lithium-metal and high-voltage transition metal cathodes, leading to continuous electrolyte degradation. This degradation has been a major hurdle in achieving the desired cycle life of over 1,000 cycles and an energy density of more than 500 Wh/kg. Despite extensive efforts by researchers globally, the interfaces between the electrolyte and electrodes have proven difficult to stabilize, unlike the case with lithium-ion batteries.
Approximately five years ago, Prof. Shuhong Jiao and her colleagues devised an electrolyte that could simultaneously stabilize the anode-electrolyte and cathode-electrolyte interfaces within lithium-metal battery cells, effectively suppressing electrolyte degradation. The unique design of this electrolyte focuses on the microscopic physicochemical processes occurring inside lithium-metal batteries, offering a new perspective on electrolyte composition and performance enhancement.
Pioneering Advances in Electrolyte Solvation Structure
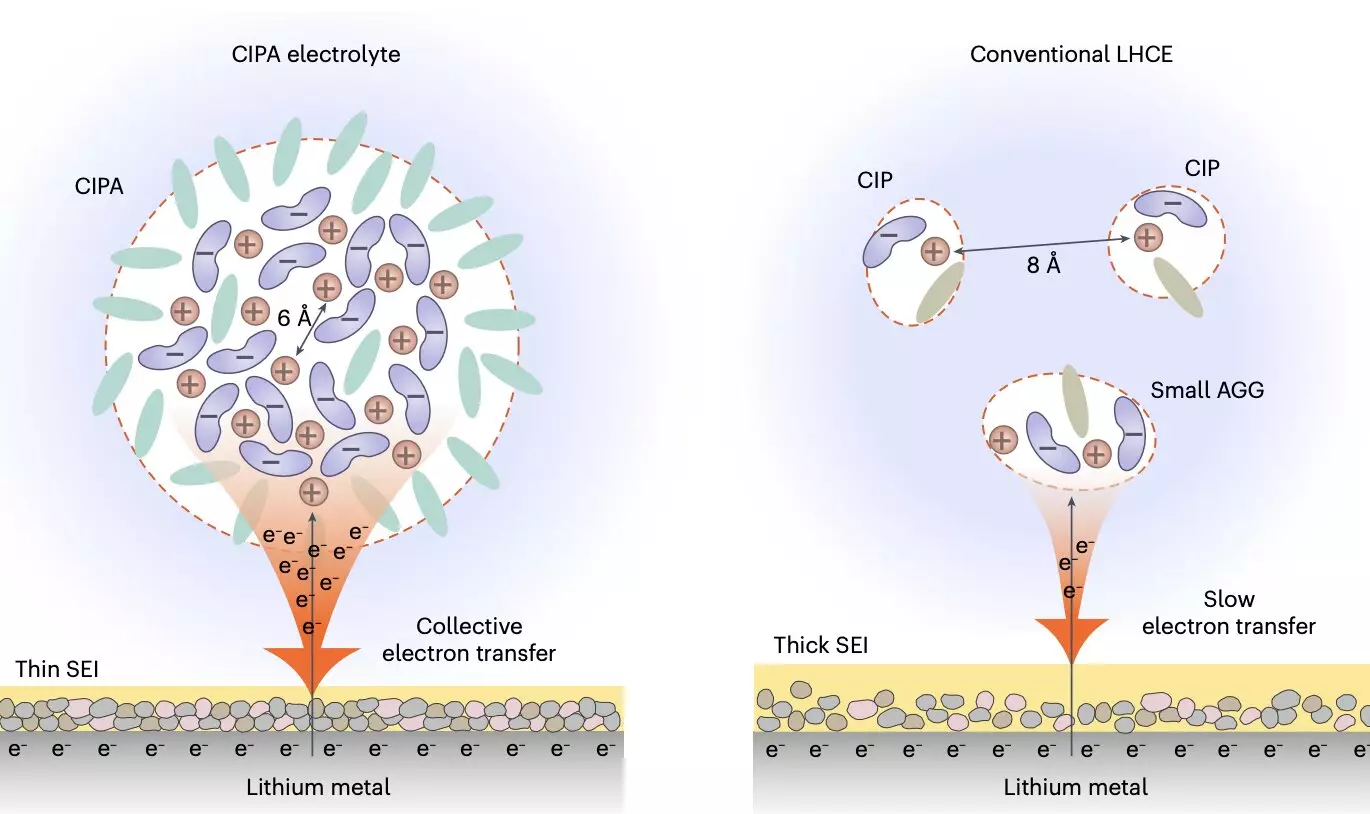
The recent study by Prof. Jiao’s research group delves into the mesoscopic level tuning of electrolyte solvation structure, specifically targeting the interaction between ion pairs that underlie the formation of the electrolyte’s aggregate structure. The design features compact ion-pair aggregates, a stark departure from the prevailing small aggregates and separate ion pairs found in existing electrolyte classes. This innovative approach has opened up a new avenue for electrolyte design in lithium-metal batteries.
Enhanced Stability and Performance through Collective Reduction
The newly designed electrolyte exhibits a collective reduction mechanism on the lithium-metal anode, resulting in the rapid decomposition of anions to form inorganic compounds and a thin, stable solid electrolyte interface (SEI). This SEI suppresses continuous electrolyte decomposition, promotes homogeneous lithium ion flux, and facilitates dendrite-free lithium deposition, ultimately enhancing the overall performance and stability of lithium-metal batteries.
The successful implementation of this groundbreaking electrolyte design has demonstrated significant improvements in energy retention and cycle stability, with promising results indicating the potential for further advancements in lithium-metal battery technology. The researchers are now looking towards extending the cycle life of lithium-metal pouch cells beyond 1,000 cycles and exploring the possibility of achieving even higher energy densities exceeding 600 Wh/kg. Collaborative efforts among researchers worldwide will be crucial in realizing the full potential of this revolutionary electrolyte design for long-lasting, high-energy lithium-metal batteries.

Leave a Reply