In a groundbreaking study recently published in Nature Communications, researchers from the Interface Science Department at the Fritz Haber Institute have made significant strides in the fight against climate change. Their work introduces a revolutionary method for understanding the mechanisms of carbon dioxide (CO2) re-utilization, leading to the production of fuels and chemicals. This breakthrough paves the way for the optimization of catalytic processes driven by renewable electricity.
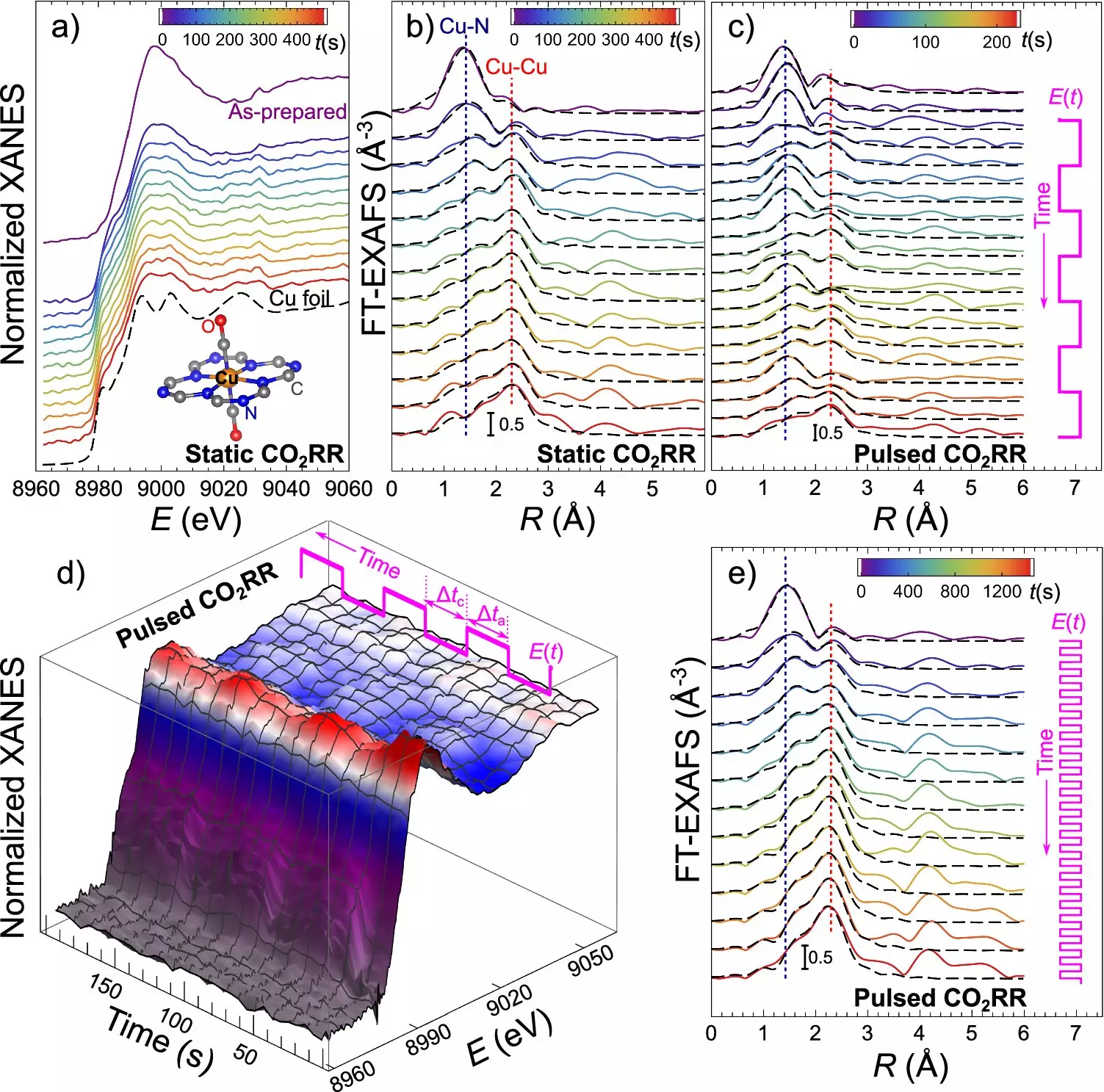
The core of this groundbreaking discovery lies in the unique properties of catalysts made of ultradispersed copper and nitrogen atoms incorporated into carbon. These catalysts exhibit the ability to dynamically transform from single atoms to small clusters and metal particles, known as nanoparticles, during the electrocatalytic CO2 reduction process. What sets this catalyst apart is its ability to reverse this transformation when the applied electrical potential is changed, providing unprecedented control over the catalyst’s structure and consequently, the outcome of the CO2RR process.
The key to the success of this innovative approach lies in the researchers’ ability to control the size and structure of the catalyst particles, addressing a major challenge in scaling up CO2RR technology for practical use. By precisely manipulating the catalyst’s state, the distribution of CO2RR products can be controlled, enabling the production of specific industrially relevant chemicals and fuels with high efficiency.
The research conducted by the team at the Fritz Haber Institute showcases a method to precisely control the distribution of CO2RR products by manipulating the catalyst’s structure. By utilizing alternating electrical pulses, the researchers can steer the sizes of formed nanoparticles, determining whether the catalyst exists mostly as single atoms, ultrasmall metal clusters, or larger metallic copper nanoparticles. Each form of the catalyst is tailored to produce different CO2RR products, with single copper atoms being efficient for hydrogen production, small clusters favoring methane, and larger nanoparticles best suited for ethylene production.
To monitor and adjust the catalyst’s transformation in real-time, the team employed operando quick X-ray absorption spectroscopy. This advanced synchrotron-based technique offers sub-second time resolution, allowing scientists to observe the catalyst as it changes during the reaction. This level of precision ensures the optimal conditions for the desired CO2RR products and provides valuable insights into catalyst behavior and structural transformations.
This study not only deepens our understanding of catalyst behavior but also sheds light on the CO2 reduction reaction, illustrating how controlling the catalyst’s structure can significantly impact the process. While the research holds promise for technological applications in greenhouse gas reduction and the production of green chemicals and fuels, its primary significance lies in advancing scientific inquiry and setting the stage for future breakthroughs in the field of catalysis.

Leave a Reply