Ammonia production is a critical process for the creation of fertilizers needed for agriculture, but the current methods are unsustainable and detrimental to the environment. Converting dinitrogen gas to ammonia requires a significant amount of energy and results in the emission of greenhouse gases, primarily carbon dioxide. With the Haber-Bosch process being the standard method for ammonia production, it is clear that a more environmentally friendly approach is needed.
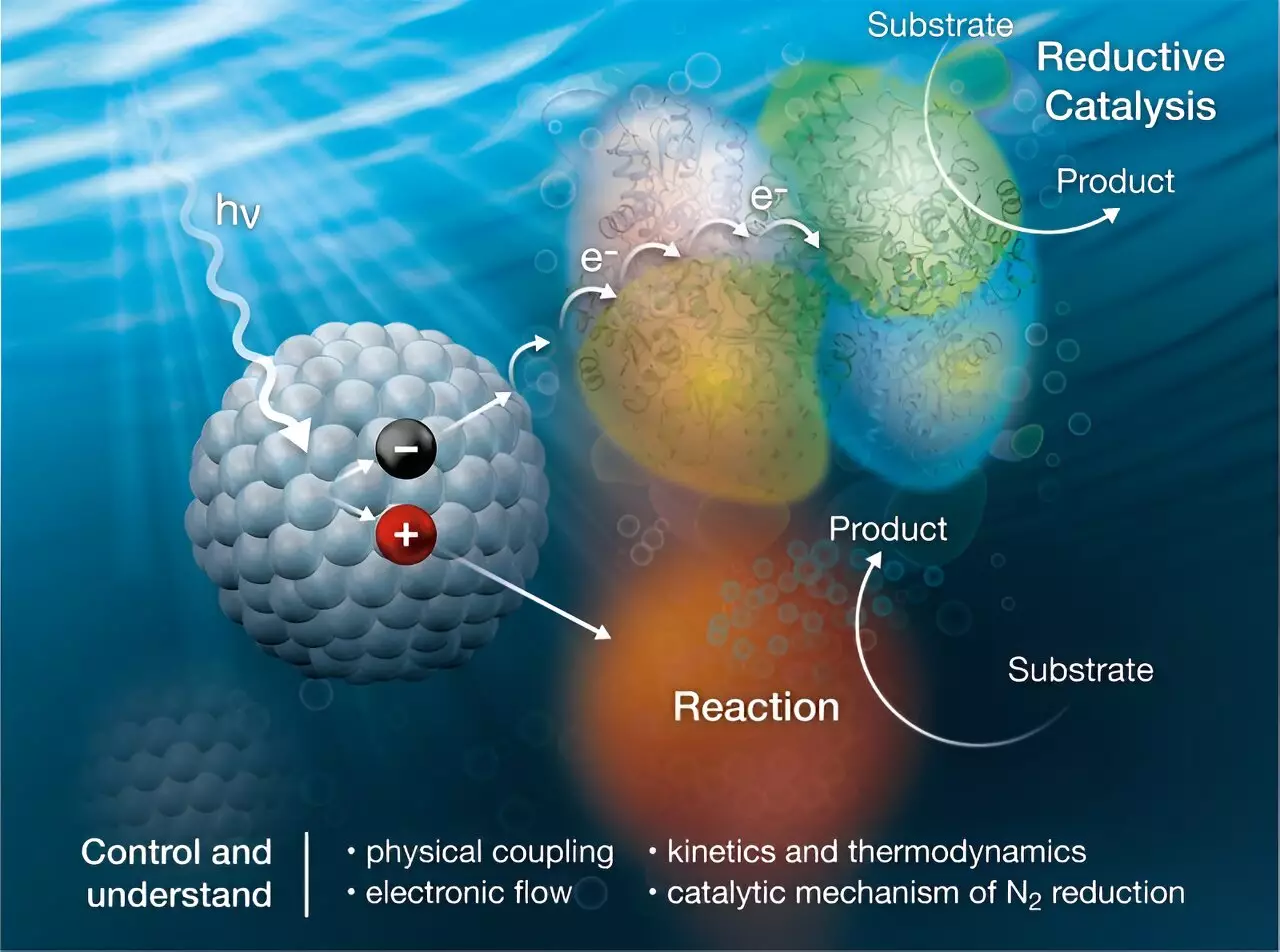
Researchers have been exploring the possibility of using sunlight energy to drive the conversion of nitrogen to ammonia, eliminating the need for ATP and reducing greenhouse gas emissions. In a recent study published in the Journal of the American Chemical Society, scientists developed a biohybrid system that combines nanocrystals with the nitrogenase enzyme to catalyze the ammonia production reaction using sunlight as the energy source.
The Role of Nanocrystals in the Process
The key innovation in this research lies in the utilization of nanocrystals to transfer charge to the nitrogenase enzyme and facilitate the ammonia production reaction. By understanding the properties of nanocrystals that allow them to bind to the enzyme, researchers gained valuable insights into the intricacies of the NH3 production process. This biohybrid approach shows promise in driving energy-demanding conversion reactions without the generation of greenhouse gases.
The new process of using sunlight to catalyze NH3 production offers several advantages over the traditional Haber-Bosch method. Not only does it eliminate the production of CO2, but it also enables the production of NH3 fertilizers in close proximity to where they will be used, reducing emissions from transportation. By coupling sunlight with the ammonia production process, researchers are paving the way for a more sustainable and environmentally friendly approach to fertilizer production.
Moving forward, further research is needed to optimize the nanocrystal/enzyme system for efficient ammonia production. By studying the mechanisms of charge transfer and reaction intermediates, researchers can continue to refine the process and develop a comprehensive kinetic model of the N2 reduction reaction. Through continued innovation and collaboration, the future of sustainable ammonia production using sunlight as an energy source looks promising.

Leave a Reply