In a groundbreaking development, researchers at Oregon State University have unveiled a material with the extraordinary ability to convert sunlight and water into clean energy. Spearheaded by Kyriakos Stylianou of the OSU College of Science, this collaborative effort has led to the creation of a photocatalyst that facilitates the rapid and efficient production of hydrogen. This hydrogen serves as a vital component in fuel cells for automobiles, as well as in the production of various chemicals such as ammonia, refining of metals, and manufacturing of plastics.
The foundation of this innovative research lies in the utilization of metal organic frameworks (MOFs), crystalline and porous materials that possess unique properties conducive to catalysis. Comprising positively charged metal ions surrounded by organic “linker” molecules, MOFs exhibit nanosized pores and adjustable structural characteristics. These frameworks can be tailored with diverse components to dictate their overall properties.
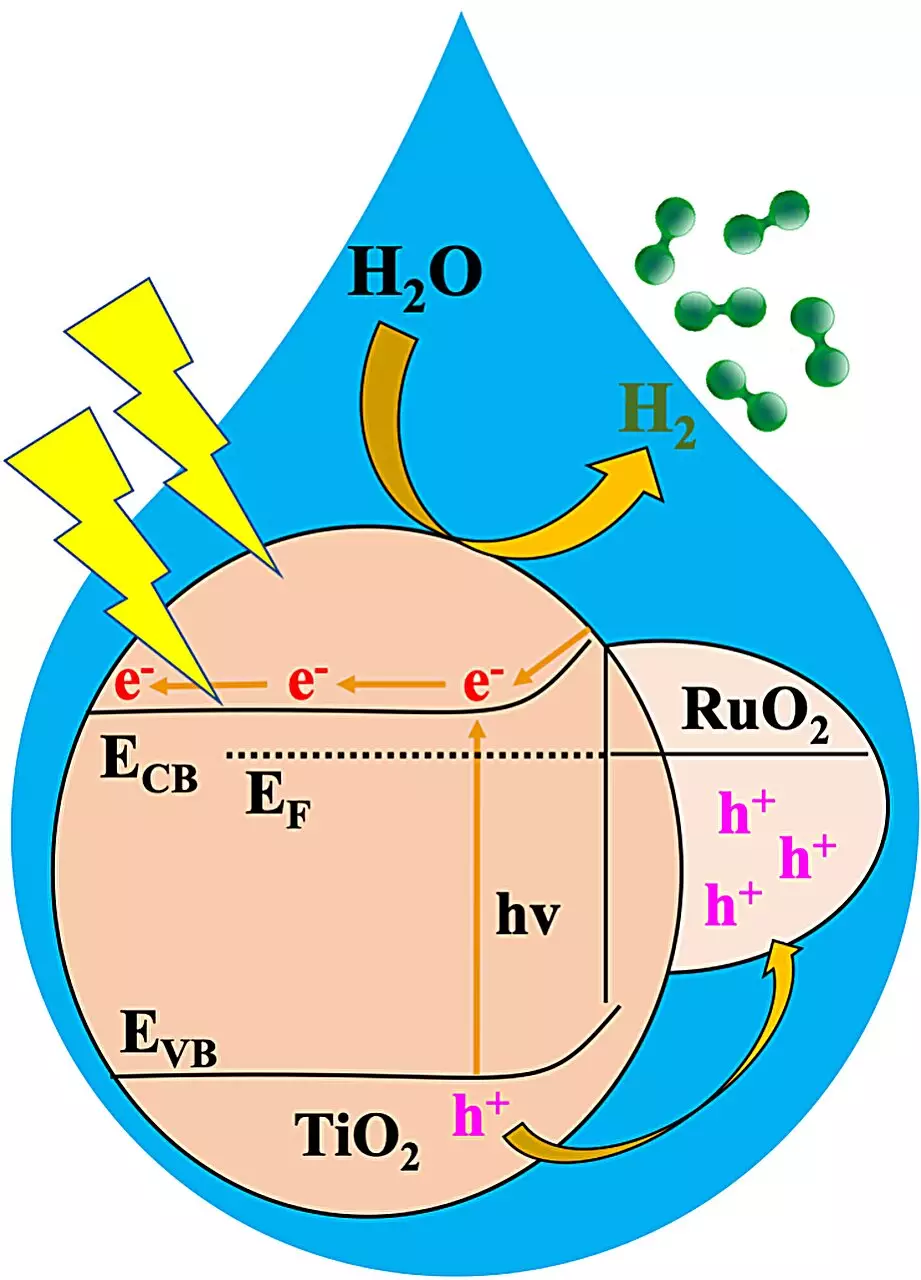
The focal point of this study revolves around the creation of a metal oxide heterojunction, named RTTA, achieved by combining ruthenium oxide and titanium oxide derived from MOFs and doped with sulfur and nitrogen. Through extensive experimentation with various RTTAs containing different amounts of the oxides, researchers identified a superior performer in RTTA-1.
RTTA-1 showcased unparalleled performance, generating over 10,700 micromoles of hydrogen in just one hour. This remarkable feat was attributed to the synergistic effects of the metal oxides’ properties and the surface properties inherited from the parent MOF, which enhanced electron transfer. The efficiency of RTTA-1 was further accentuated by its impressive quantum yield rate of 10%.
The advent of MOF-derived metal oxide heterojunctions as efficient photocatalysts represents a significant stride towards sustainable energy solutions. By harnessing sunlight to split water and produce hydrogen, this innovative approach mitigates the environmental impact associated with traditional hydrogen production methods.
Notably, the conventional method of deriving hydrogen from natural gas via methane-steam reforming is considerably more carbon-intensive than the catalytic process facilitated by MOF-derived catalysts. With the cost of green hydrogen currently sitting at around $5 per kilogram, the sustainability and affordability of hydrogen production are critical considerations in the transition towards cleaner energy alternatives.
The development of MOF-derived catalysts heralds a new era in clean energy production. By leveraging the inherent properties of metal organic frameworks to create efficient photocatalysts like RTTA-1, researchers have unlocked a pathway towards sustainable and cost-effective hydrogen production. As we strive to combat greenhouse gas emissions and address the pressing challenges of climate change, innovations in energy production such as this represent a beacon of hope for a greener future.

Leave a Reply