Perfluoroalkyl substances (PFAS), known as “forever chemicals,” have become a significant environmental and health concern. These chemicals, including perfluorinated polymers or PFs, have been extensively used since the introduction of Teflon in 1938 due to their exceptional stability and resistance to water and heat. While these properties make PFAS ideal for various applications like cookware and firefighting foam, their stability also leads to a major problem. PFAS do not readily biodegrade, resulting in their accumulation in water, soil, and even human bodies. The presence of PFAS has been linked to carcinogenic effects and hormonal disruptions, raising alarms about the widespread contamination they cause.
Despite efforts to phase out PFAS production, their treatment remains a significant challenge. These chemicals decompose only at extremely high temperatures exceeding 400°C, making conventional methods ineffective in breaking them down. The disposal of PFAS-containing products in landfills poses a potential risk of future contamination, highlighting the urgent need for innovative treatment solutions that are both effective and sustainable.
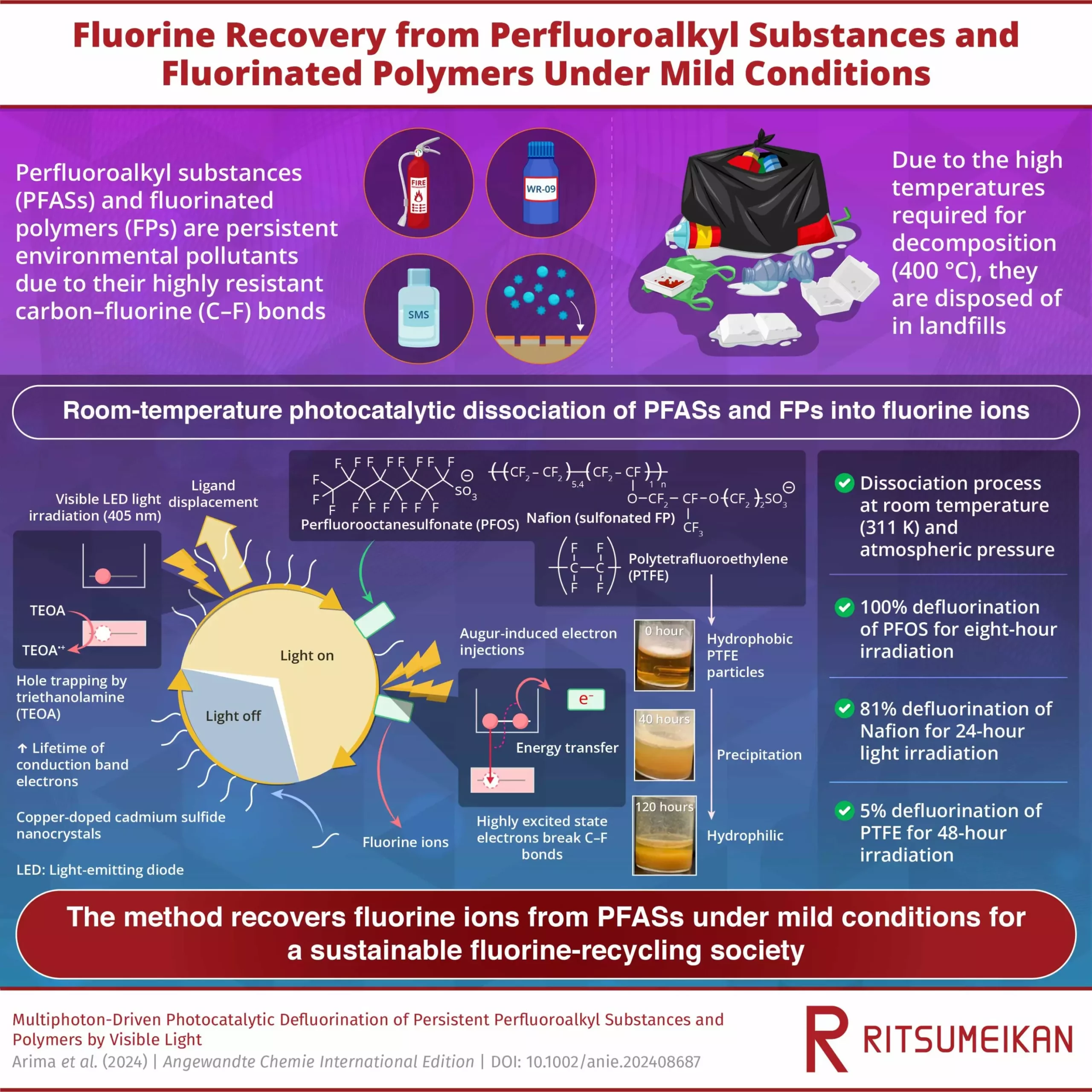
Researchers at Ritsumeikan University have introduced a groundbreaking room-temperature defluorination method for PFAS treatment that could revolutionize the way these chemicals are handled. Published in the journal Angewandte Chemie International Edition, their study presents a photocatalytic approach that utilizes visible light to break down PFAS and other fluorinated polymers (FPs) at room temperature, transforming them into fluorine ions. The researchers achieved 100% defluorination of perfluorooctanesulfonate (PFOS) within just 8 hours of light exposure, showcasing the efficiency of this method in decomposing PFAS under gentle conditions.
The proposed method involves irradiating visible LED light onto cadmium sulfide (CdS) nanocrystals and copper-doped CdS (Cu-CdS) nanocrystals in a solution containing PFAS, FPs, and triethanolamine (TEOA). This irradiation generates electrons with a high reduction potential, which effectively break down the strong carbon-fluorine bonds present in PFAS molecules. By leveraging the photocatalytic properties of semiconductor nanocrystals, the researchers were able to initiate a series of reactions that led to the removal of fluorine ions from the PFAS molecules, thereby achieving defluorination.
This innovative defluorination method not only offers a promising solution for the effective treatment of PFAS but also contributes significantly to the establishment of a sustainable fluorine-recycling society. By recovering fluorine from waste PFAS, industries that rely on fluorine, such as pharmaceuticals and clean energy technologies, can reduce their dependence on fluorine production, paving the way for a more environmentally friendly recycling process. This technique represents a crucial step towards the development of recycling technologies for fluorine elements, supporting the growth of a prosperous and sustainable society.
The room-temperature defluorination method proposed by the researchers at Ritsumeikan University marks a significant advancement in PFAS treatment, offering a safe, efficient, and environmentally friendly approach to addressing the challenges posed by “forever chemicals.” This breakthrough not only holds promise for mitigating the harmful effects of PFAS contamination but also opens up new possibilities for sustainable recycling practices that benefit various industries and contribute to a greener future.

Leave a Reply