Chemicals play a vital role in our everyday lives, from consumer products to industrial applications. However, the process of converting chemicals often requires a significant amount of energy from non-renewable sources. The chemicals and petrochemicals industries are responsible for around 40% of all industrial energy use and emissions in the United States. This has led to a growing interest in using light to drive chemical reactions, as light, especially sunlight, is a free and abundant source of energy. Researchers are exploring innovative ways to leverage light for chemistry to increase efficiency and reduce environmental impact.
A research team at the University of Illinois Urbana-Champaign has made significant strides in understanding the use of light for chemical reactions. They have discovered a novel mechanism of charge transfer that is faster and more efficient than traditional methods. By utilizing plasmonic gold particles, which are incredibly small at 1/1000th the width of a human hair, the researchers were able to transfer charge to a semiconductor material with titanium oxide shells. This innovative approach has the potential to revolutionize light-harvesting technology for generating electricity or driving chemical reactions.
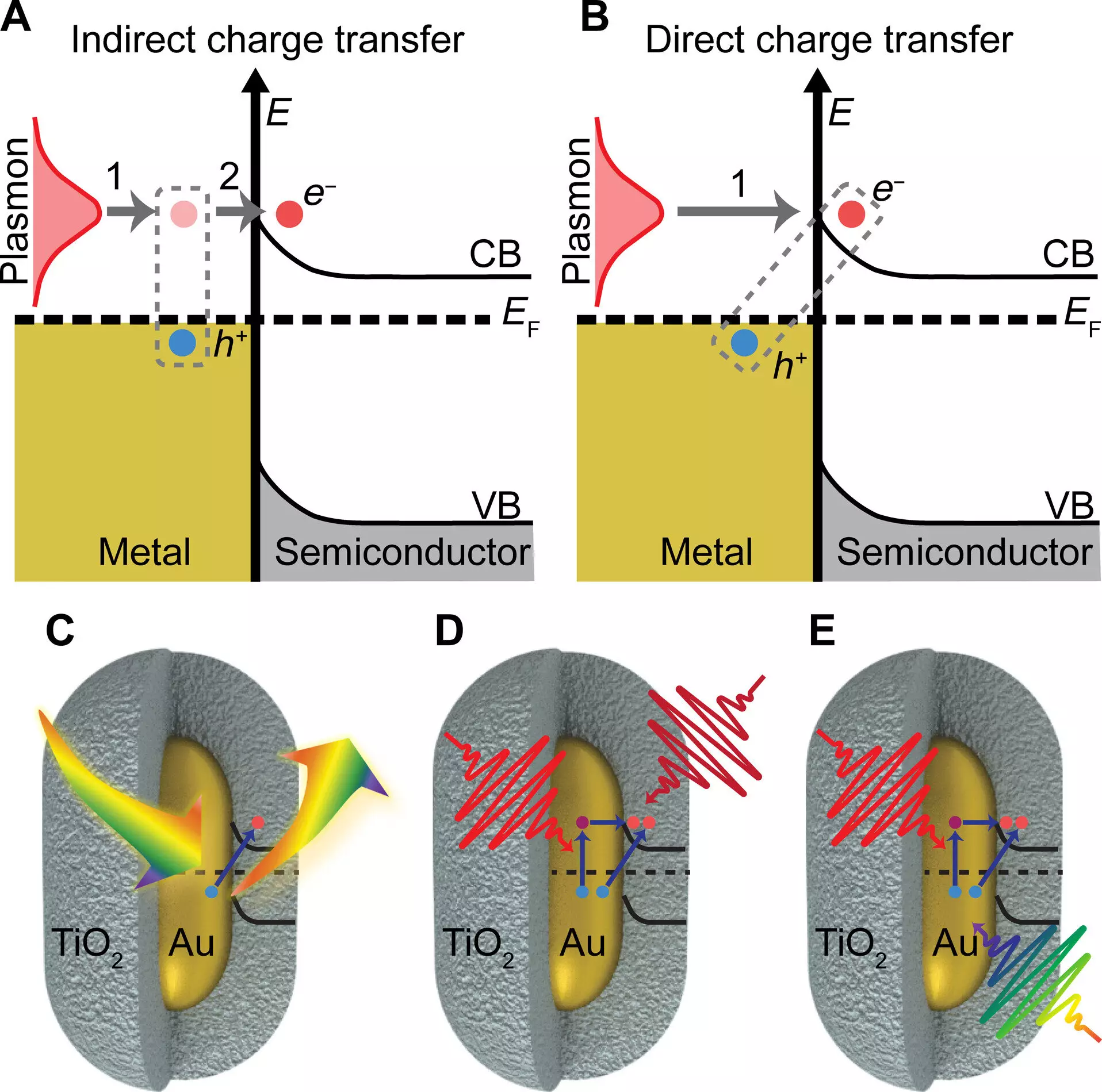
One of the key findings of the research is the enhanced charge transfer efficiency achieved through the use of gold nanoparticles. These nanoparticles have unique properties that allow them to absorb light more effectively than other particles of the same size. By exciting the plasmon of the gold particles with light, the researchers were able to significantly boost charge transfer to the semiconductor material. This discovery opens up new possibilities for designing more efficient devices that convert light energy into electrical or chemical energy.
The study also revealed that the gold particles exhibit higher charge transfer efficiency when exposed to light of a specific color that matches the particles. This correlation between light color and charge transfer efficiency highlights the importance of tuning the properties of the nanoparticles for optimal performance. By focusing on the plasmon of the gold particles, the researchers were able to create a shortcut path to the charge separated state, bypassing unwanted heating effects. This innovative approach paves the way for more effective utilization of light in driving chemical reactions.
The research team’s work sheds light on a long-neglected process known as chemical interface damping of plasmons, which was proposed in the 1990s. This process, which involves the direct plasmon-induced charge transfer, has significant implications for the field of plasmonics and plasmonic photocatalysis. The researchers employed a variety of techniques, including imaging and spectroscopy, to confirm their results and gain a comprehensive understanding of the chemical interface damping phenomenon. This multidisciplinary approach underscores the importance of collaboration and interdisciplinary research in advancing the field of chemistry.
Overall, the use of light for driving chemical reactions holds great promise for increasing energy efficiency, reducing environmental impact, and advancing the field of chemistry. By leveraging the unique properties of plasmonic gold particles and optimizing light absorption, researchers are paving the way for a more sustainable and efficient approach to chemical processes. This groundbreaking research opens up new avenues for harnessing light energy to power the chemical reactions that drive our modern world.

Leave a Reply