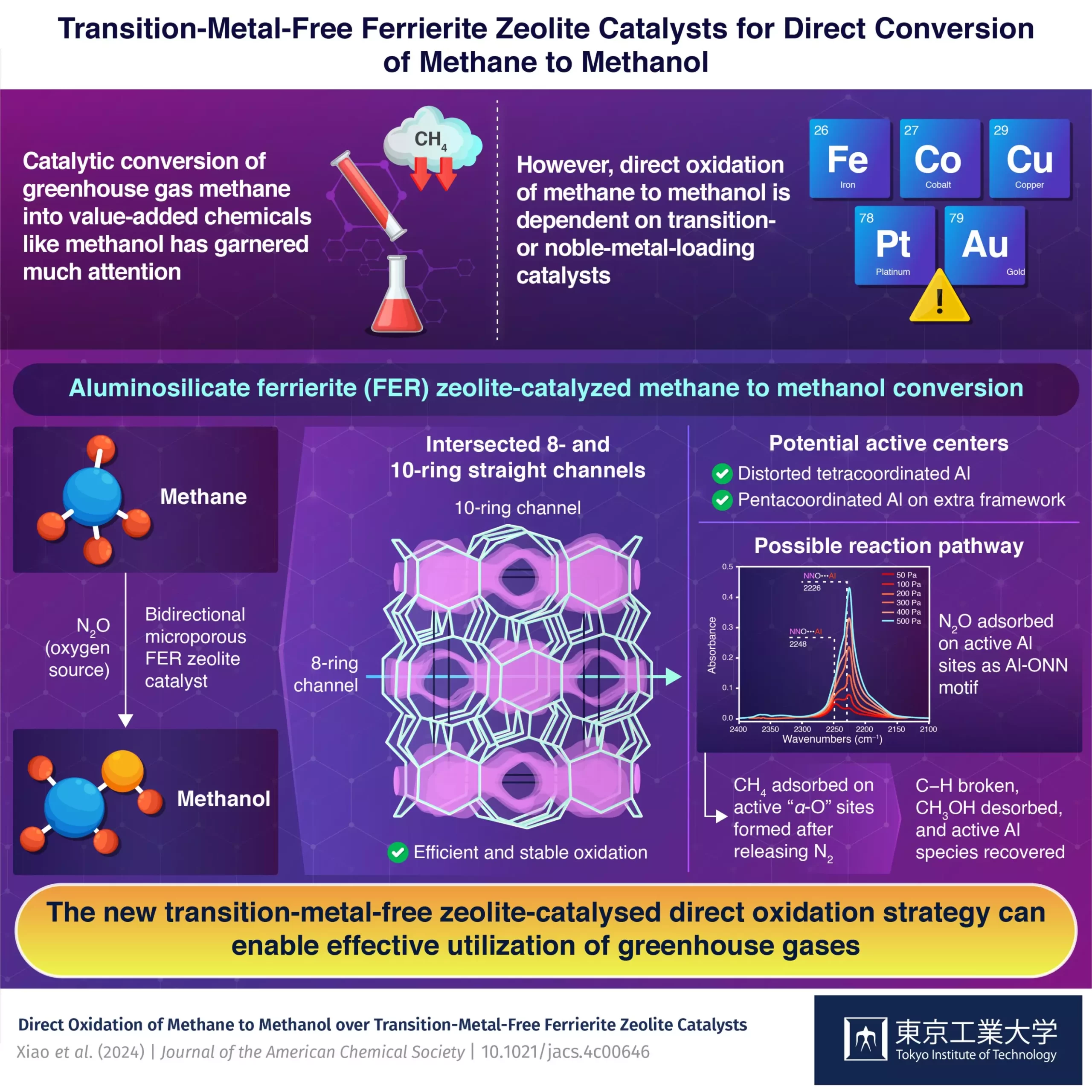
The waste-to-wealth movement has sparked a wave of innovation in the field of technology aimed at converting greenhouse gases into valuable materials. One technology that has garnered significant attention is the catalytic conversion of methane into methanol, a versatile industrial solvent and raw material for chemical synthesis. The traditional industrial process for this conversion is known to be highly energy and resource-intensive, prompting scientists to explore alternative, more sustainable methods.
In a recent study published in the Journal of the American Chemical Society, a team of researchers led by Associate Professor Toshiyuki Yokoi and Assistant Professor Peipei Xiao from the Nanospace Catalysis Unit at the Institute of Innovative Research at Tokyo Institute of Technology, Japan, made a groundbreaking discovery. They identified transition-metal-free aluminosilicate ferrierite (FER) zeolite as a novel catalyst for the direct oxidation of methane to methanol using nitrous oxide as the source of oxygen.
Redefining Catalyst Composition
Unlike conventional catalyst systems that rely on rare and expensive transition or noble metals, the FER zeolite offers a unique solution by utilizing only main group elements. This pioneering approach challenges the traditional norms of catalyst composition and opens up new possibilities for sustainable methane conversion processes.
To gain insights into the active sites within the zeolite catalyst, the research team conducted a series of tests including Fourier transform infrared spectroscopy (FTIR) and magnetic resonance spectroscopy. These tests revealed the presence of distorted tetracoordinated Al in the framework and pentacoordinated Al in the extra framework, highlighting their potential as active centers for catalytic reactions.
The transition-metal-free zeolite catalyst demonstrated exceptional performance, achieving a methanol production rate of 305 μmol g−1 min−1 with an impressive 89% methanol and 10% dimethyl ether selectivity. These results surpassed the performance of many transition metal-loaded catalysts, showcasing the potential of this innovative approach for sustainable methane conversion.
The implications of this research extend beyond the laboratory, offering a promising avenue for the development of environmentally-friendly methane conversion processes. By reducing greenhouse gas emissions and enabling the synthesis of valuable chemicals from waste materials, the transition-metal-free aluminosilicate zeolite catalyst represents a significant step towards a more sustainable future.
Through their pioneering work, Yokoi, Xiao, and their team have not only advanced the field of catalysis but have also laid the foundation for a more sustainable approach to methane conversion. This research paves the way for further exploration and innovation in the realm of transition-metal-free catalysts, highlighting the potential for creating a positive environmental impact while driving economic growth through resource utilization.

Leave a Reply